Key Points
-
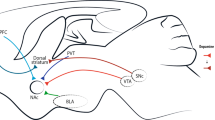
Addiction — the loss of control over drug use — involves stable brain changes that are responsible for the long-lasting nature of the behavioural abnormalities. Although drugs of abuse comprise chemically divergent molecules, their actions in the brain converge in some common points. In particular, all of them lead to the activation of the mesolimbic dopamine system — a main reward system of the brain. One likely mechanism of addiction is that repeated stimulation of the mesolimbic dopamine system leads to marked alterations in reinforcement mechanisms and motivational state.
-
Different molecular modifications have been put forward to explain the long-lasting nature of addiction. For example, transcriptional modifications are thought to be crucial for the development of addictive states. The two main transcriptional regulators known to change upon repeated drug exposure are CREB and ΔFosB.
-
Increased transcription of the CREB gene accompanies the upregulation of the cAMP pathway known to occur in certain brain regions after repeated drug intake. Upregulation of the cAMP pathway and CREB might mediate a homeostatic adaptation that diminishes further drug responsiveness. However, as the increased levels of CREB have a relatively short life, additional mechanisms are thought to underlie longer-lasting manifestations of addiction.
-
The levels of ΔFosB, another transcriptional regulator, are also increased after administration of drugs of abuse. As the half-life of this molecule is very long, it provides a molecular mechanism of addiction based in the stability of the protein by which drug-induced changes in gene expression can persist long after drug intake stops.
-
Other mechanisms independent of transcription might contribute to the long-term plastic changes underlying addiction. For instance, reduced protein degradation and the regulation of receptor sensitivity are two modifications known to occur after drug administration. Similarly, structural changes in the neurons of the mesolimbic dopamine system and their targets, such as increased spine density in the nucleus accumbens, are known to accompany repeated drug exposure.
-
There are many parallels between the molecular changes underlying addiction and those related to other forms of brain plasticity such as learning and memory. From a behavioural perspective, certain features of addiction, such as the ability of drug-associated cues to induce relapse, have been described as forms of memory. Also, activation of the cAMP pathway and of CREB-mediated transcription has been observed in forms of synaptic plasticity believed to constitute cellular correlates of memory. As a result, the key future challenges in the addiction and the memory fields are equivalent to a large extent.
Abstract
Studies of human addicts and behavioural studies in rodent models of addiction indicate that key behavioural abnormalities associated with addiction are extremely long lived. So, chronic drug exposure causes stable changes in the brain at the molecular and cellular levels that underlie these behavioural abnormalities. There has been considerable progress in identifying the mechanisms that contribute to long-lived neural and behavioural plasticity related to addiction, including drug-induced changes in gene transcription, in RNA and protein processing, and in synaptic structure. Although the specific changes identified so far are not sufficiently long lasting to account for the nearly permanent changes in behaviour associated with addiction, recent work has pointed to the types of mechanism that could be involved.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Koob, G. F. & Nestler, E. J. Neurobiology of drug addiction . J. Neuropsychiat. Clin. Neurosci. 9, 482 –497 (1997). PubMed
Wise, R. A. Drug-activation of brain reward pathways. Drug Alcohol Dependence 51, 13–22 ( 1998). PubMed
Robinson, T. E. & Berridge, K. C. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95, S91–S117 ( 2000).
Koob, G. F., Sanna, P. P. & Bloom, F. E. Neuroscience of addiction. Neuron 21, 467–476 (1998).
Robbins, T. W. & Everitt, B. J. Neurobehavioural mechanisms of reward and motivation. Curr. Opin. Neurobiol. 6, 228–236 (1996).
Self, D. W. & Nestler, E. J. Relapse to drug seeking: neural and molecular mechanisms. Drug Alcohol Dependence 51 , 49–60 (1998). PubMed
Shaham, Y., Erb, S. & Stewart, J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res. Rev. 33, 13– 33 (2000).Relapse to drug use after some period of abstinence is one of the cardinal features of addiction. An important goal of current drug-abuse research is to understand the neurobiological mechanisms of relapse and to develop improved treatments. Shaham and colleagues provide an outstanding review of this field, and highlight the considerable advances that have been made in developing better animal models of relapse and in using these models to study the underlying neurobiology.
Nestler, E. J., Hope, B. T. & Widnell, K. L. Drug addiction: a model for the molecular basis of neural plasticity. Neuron 11, 995– 1006 (1993).
Berke J. D. & Hyman, S. E. Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25, 515– 532 (2000).
Carey, M. & Smale, S. T. Transcriptional Regulation in Eukaryotes (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 2000).
Nestler, E. J. & Aghajanian, G. K. Molecular and cellular basis of addiction. Science 278, 58–63 (1997).
Shaywitz, A. J. & Greenberg, M. E. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals . Annu. Rev. Biochem. 68, 821– 861 (1999).
De Cesare, D. & Sassone-Corsi, P. Transcriptional regulation by cyclic AMP-responsive factors. Prog. Nucl. Acid Res. Mol. Biol. 64, 343–369 ( 2000). PubMed
Guitart, X. et al. Regulation of CREB phosphorylation by acute and chronic morphine in the rat locus coeruleus. J. Neurochem. 5, 1168–1171 (1992). PubMed
Sharma, S. K., Klee, W. A. & Nirenberg, M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc. Natl Acad. Sci. USA 72, 3092–3096 (1975).
Terwilliger, R. Z., Beitner-Johnson, D., Sevarino, K. A., Crain, S. M. & Nestler, E. J. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 548, 100–110 (1991).
Unterwald, E. M., Cox, B. M., Kreek, M. J., Cote, T. E. & Izenwasser, S. Chronic repeated cocaine administration alters basal and opioid-regulated adenylyl cyclase activity. Synapse 15, 33–38 (1993).
Bonci, A. & Williams, J. T. Increased probability of GABA release during withdrawal from morphine. J. Neurosci. 17, 796–803 (1997).
Jolas, T., Nestler, E. J. & Aghajanian, G. K. Chronic morphine increases GABA tone on serotonergic neurons of the dorsal raphe nucleus: association with an upregulation of the cyclic AMP pathway. Neuroscience 95, 433 –443 (2000).
Chakrabarti, S., Rivera, M., Yan, S. Z., Tang, W. J. & Gintzler, A. R. Chronic morphine augments Gβγ/Gsα stimulation of adenylyl cyclase: relevance to opioid tolerance. Mol. Pharmacol. 54, 655–662 (1998).
Lane-Ladd, S. B. et al. CREB (cAMP response element binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J. Neurosci. 17, 7890 –7901 (1997).
Widnell, K. L., Russell, D. & Nestler, E. J. Regulation of cAMP response element binding protein in the locus coeruleus in vivo and in a locus coeruleus-like (CATH.a) cell line in vitro. Proc. Natl Acad. Sci. USA 91, 10947–10951 (1994).
Coven, E. et al. Cell-type specific regulation of CREB gene expression: mutational analysis of CREB promoter activity. J. Neurochem. 71 , 1865–1874 (1998).
Chao, J. R., Ni, Y. G., Chen, J. S., Rahman, Z., & Nestler, E. J. Characterization of the mouse adenylyl cyclase type VIII gene promoter: activiation by cAMP. Soc. Neurosci. Abstr. 26, 125 (2000).
Maldonado, R. et al. Reduction of morphine abstinence in mice with a mutation in the gene encoding CREB. Science 273, 657 –659 (1996).
Chen, J. S. et al. Transgenic animals with inducible, targeted gene expression in brain. Mol. Pharmacol. 54, 495– 503 (1998).
Cole, R. L., Konradi, C., Douglass, J. & Hyman, S. E. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron 14, 813–823 (1995).
Turgeon, S. M., Pollack, A. E. & Fink, J. S. Enhanced CREB phosphorylation and changes in c-Fos and FRA expression in striatum accompany amphetamine sensitization. Brain Res. 749, 120–126 (1997).
Shaw, T. Z. et al. Upregulation of CRE-mediated transcription during naltrexone-precipitated opiate withdrawal. Soc. Neurosci. Abstr. 25, 422 (1999).
Carlezon, W. A. Jr et al. Regulation of cocaine reward by CREB. Science 282, 2272–2275 ( 1998).
Barrot, M., Olivier, J. D. A., Zachariou, V., Neve, R. L. & Nestler, E. J. Influence of CREB in the nucleus accumbens shell on the sensitivity to aversive and nociceptive stimuli. Soc. Neurosci. Abstr. 26, 485 ( 2000).
Self, D. W. et al. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior . J. Neurosci. 18, 1848– 1859 (1998).
Nestler, E. J. Genes and addiction. Nature Genet. 26, 277 –281 (2000).
Hyman, S. E. Addiction to cocaine and amphetamine. Neuron 16, 901–904 (1996).
Kreek, M. J. Opiate and cocaine addictions: challenge for pharmacotherapies. Pharmacol. Biochem. Behav. 57, 551– 569 (1997).
Shippenberg, T. S. & Rea, W. Sensitization to the behavioral effects of cocaine: modulation by dynorphin and kappa-opioid receptor agonists. Pharmacol. Biochem. Behav. 57, 449–455 (1997).
Daunais, J. B. & McGinty, J. F. Cocaine binges differentially alter striatal preprodynorphin and zif/268 mRNAs. Mol. Brain Res. 29, 201–210 (1995).
Spangler, R. et al. Regulation of kappa opioid receptor mRNA in the rat brain by 'binge' pattern cocaine administration and correlation with preprodynorphin mRNA. Mol. Brain Res. 38, 71– 76 (1996).
Morgan, J. I. & Curran, T. Immediate-early genes: ten years on. Trends Neurosci. 18, 66– 67 (1995).
Hope, B. T. et al. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments . Neuron 13, 1235–1244 (1994).
Moratalla, R., Elibol, B., Vallejo, M. & Graybiel, A. M. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron 17 , 147–156 (1996).
Kelz, M. B. & Nestler, E. J. ΔFosB: A molecular switch underlying long-term neural plasticity. Curr. Opin. Neurol. 13, 715–720 (2000).
Hiroi, N. & Graybiel, A. M. Atypical and typical neuroleptic treatments induce distinct programs of transcription factor expression in the striatum. J. Comp. Neurol. 374, 70– 83 (1996).
Atkins, J. et al. Region-specific induction of ΔFosB by repeated administration of typical versus atypical antipsychotic drugs. Synapse 33, 118–128 (1999).
Kelz, M. B. et al. Expression of the transcription factor ΔFosB in the brain controls sensitivity to cocaine. Nature 401, 272–276 (1999).The authors used the tetracycline-driven gene expression system to generate bitransgenic mice that overexpress ΔFosB selectively within the nucleus accumbens and dorsal striatum in an inducible manner. These mice overcome limitations of conventional transgenics owing to the inducibility and tissue selectivity of transgene expression. The authors show that induction of ΔFosB in these brain regions of adult animals markedly increases the sensitivity to the locomotor-activating and rewarding properties of cocaine.
Whisler, K., Kelz, M. B., Chen, J. S., Nestler, E. J. & Self, D. W. Effects of conditional overexpression of ΔFosB in nucleus accumbens on cocaine self-administration and relapse to cocaine-seeking behavior. Soc. Neurosci. Abstr. 25, 811 (1999).
Peakman, M.-C. et al. Inducible brain-region specific expression of Δc-Jun in transgenic mice decreases sensitivity to cocaine. Soc. Neurosci. Abstr. 26, 124 (2000).
Hiroi, N. et al. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine's psychomotor and rewarding effects. Proc. Natl Acad. Sci. USA 94, 10397 –10402 (1997).
White, F. J., Hu, X.-T., Zhang, X.-F. & Wolf, M. E. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J. Pharmacol. Exp. Ther. 273, 445–454 (1995).
O'Donovan, K. J., Tourtellotte, W. G., Millbrandt, J. & Baraban, J. M. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 22, 167–173 (1999).
Mackler, S. A. et al. NAC-1 is a brain POZ/BTB protein that can prevent cocaine-induced sensitization in the rat. J. Neurosci. 20, 6210–6217 (2000). The authors show that overexpression of Nac-1, a protein regulated by cocaine administration in the nucleus accumbens, antagonizes locomotor sensitization induced by repeated cocaine administration. Thus, cocaine induction of Nac-1, like cocaine activation of CREB, would seem to represent a homeostatic adaptation that serves to diminish responsiveness to subsequent drug exposures.
Piazza, P. V. & Le Moal, M. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res. Rev. 25, 359–372 (1997).
Tasken, K. et al. Structure, function, and regulation of human cAMP-dependent protein kinases. Adv. Second Messenger Phosphoprotein Res. 31, 191–204 (1997).
Boundy, V. A., Chen, J. & Nestler, E. J. Regulation of cAMP-dependent protein kinase subunit expression in CATH.a and SH-SY5Y cells. J. Pharmacol. Exp. Ther. 286, 1058–1065 ( 1998).
Lefkowitz, R. J. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J. Biol. Chem. 273, 18677–18680 (1998).
Whistler, J. L., Chuang, H. H., Chu, P., Jan, L. Y. & Von Zastrow, M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction . Neuron 23, 737–746 (1999).Tolerance to the analgesic effects of opiates is an impediment in the treatment of chronic pain syndromes but the molecular basis of tolerance has remained obscure. By using tagged opioid receptors in cell culture, this study shows that opioid-receptor tolerance probably involves internalization of the receptors and provides evidence for the role of dynamin-dependent endocytosis in this process. The study also establishes clear differences between endogenous opioid peptides versus opiate drugs, both of which are receptor agonists, in eliciting receptor internalization .
Ferguson, S. S., Zhang, J., Barak, L. S. & Caron, M. G. Molecular mechanisms of G protein-coupled receptor desensitization and resensitization. Life Sci. 62, 1561–1565 (1998).
Zaki, P. A., Keith, D. E. Jr, Brine, G. A., Carroll, F. I. & Evans, C. J. Ligand-induced changes in surface mu-opioid receptor number: relationship to G protein activation? J. Pharmacol. Exp. Ther. 292, 1127– 1134 (2000).
Bohn, L. M. et al. Enhanced morphine analgesia in mice lacking β-arrestin-2 . Science 286, 2495–2498 (1999).
Bohn, L. M. et al. μ-Opioid receptor desensitization by β-arrestin-2 determines morphine tolerance but not dependence. Nature 408, 720–723 (2000). Mice lacking β-arrestin-2 show reduced tolerance to the analgesic effects of opiates after repeated drug administration, but no difference in the degree of physical dependence as compared to controls. This is the best evidence so far that the G-protein-receptor kinase–arrestin system, which is known to mediate desensitization of many types of G-protein-coupled receptors, is also crucial in opioid-receptor tolerance. In addition, the results further clarify the partly distinct mechanisms that underlie tolerance (G-protein-receptor kinase–arrestin system) versus physical dependence (upregulation of the cAMP pathway).
Terwilliger, R. Z., Ortiz, J., Guitart, X. & Nestler, E. J. Chronic morphine administration increases β-adrenergic receptor kinase (βARK) levels in the rat locus coeruleus. J. Neurochem. 63, 1983–1986 (1994).
Sklair-Tavron, L. et al. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc. Natl Acad. Sci. USA 93, 11202–11207 (1996).
Robinson, T. E. & Kolb, B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J. Neurosci. 17, 8491–8497 (1997).
Robinson, T. E. & Kolb, B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine . Eur. J. Neurosci. 11, 1598– 1604 (1999).This study and reference 63 were the first to show that chronic administration of cocaine or the related psychostimulant amphetamine causes alterations in dendritic morphology in specific brain regions. The increases in dendritic length, branch points, and spine density observed in medium spiny neurons of the nucleus accumbens and in pyramidal cells of the prefrontal cortex persist for at least one month after the last drug exposure. Whereas the functional consequences of these changes remain unknown, they could mediate the long-lived sensitization that is observed in behavioural responses to these drugs.
Eisch, A. J., Barrot, M., Schad, C. A., Self, D. W. & Nestler, E. J. Opiates inhibit neurogenesis in the adult rat hippocampus . Proc. Natl Acad. Sci. USA 97, 7579– 7584 (2000).
Gage, F. H. Mammalian neural stem cells. Science 287, 1433–1438 (2000).
Fuchs, E. & Gould, E. Mini-review: in vivo neurogenesis in the adult brain: regulation and functional implications. Eur. J. Neurosci. 12, 2211–2214 (2000).
Horger, B. A. et al. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J. Neurosci. 19, 4110–4122 (1999).
Pierce, R. C., Pierce-Bancroft, A. F. & Prasad, B. M. Neurotrophin-3 contributes to the initiation of behavioral sensitization to cocaine by activating the Ras/Mitogen-activated protein kinase signal transduction cascade. J. Neurosci. 19, 8685–8695 (1999).
Messer, C. J. et al. Role of GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron 26, 247– 257 (2000).The authors provide evidence for a role of glial-cell-derived neurotrophic factor (GDNF) in mediating the adaptations to chronic opiate and cocaine administration. GDNF attenuates several biochemical adaptations that are normally induced in the ventral tegmental area (VTA) and the nucleus accumbens (NAc) after repeated opiate or cocaine exposure, as well as certain behavioural responses to the drugs. By contrast, biochemical and behavioural responses to cocaine are enhanced both by an antibody that neutralizes the activity of endogenous GDNF, and by knockout of the GDNF gene. Moreover, chronic opiate or cocaine administration reduces the activity of Ret, the tyrosine kinase that mediates the effects of GDNF. These data imply a feedback loop, whereby chronic drug exposure attenuates GDNF signalling, which disrupts the normal influence that GDNF exerts on the VTA–NAc pathway leading to sensitized responses to the drugs.
Wolf, D. H., Numan, S., Nestler, E. J. & Russell, D. S. Regulation of phospholipase Cγ in the mesolimbic dopamine system by chronic morphine administration. J. Neurochem. 73, 1520–1528 (1999).
Berhow, M. T., Hiroi, N. & Nestler, E. J. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J. Neurosci. 16, 4707–4715 (1996).
Flores, C., Rodaros, D. & Stewart, J. Long-lasting induction of astrocytic basic fibroblast growth factor by repeated injections of amphetamine: blockade by concurrent treatment with a glutamate antagonist. J. Neurosci. 18, 9547–9555 (1998).
Bibb, J. A. et al. Cdk5 regulates action of chronic cocaine. Nature (in the press).
Chen, J. S. et al. Induction of cyclin-dependent kinase 5 in hippocampus by chronic electroconvulsive seizures: Role of ΔFosB. J. Neurosci. 20, 8965–8971 (2000 ).
Martin, K. C. & Kandel, E. R. Cell adhesion molecules, CREB, and the formation of new synaptic connections. Neuron 17, 567–570 (1996).
Silva, A. J. & Murphy, G. G. cAMP and memory: a seminal lesson from Drosophila and Aplysia. Brain Res. Bull. 50, 441–442 (1999).
Deisseroth, K., Bito, H., Schulman, H. & Tsien, R. W. Synaptic plasticity: A molecular mechanism for metaplasticity. Curr. Biol. 5, 1334–1338 (1995).
Yin, J. C. & Tully, T. CREB and the formation of long-term memory. Curr. Opin. Neurobiol. 6, 264– 268 (1996).
Schuman, E. M. Neurotrophin regulation of synaptic transmission. Curr. Opin. Neurobiol. 9, 105–109 ( 1999).
Korte, M., Staiger, V., Griesbeck, O., Thoenen, H. & Bonhoeffer, T. The involvement of brain-derived neurotrophic factor in hippocampal long-term potentiation revealed by gene targeting experiments. J. Physiol. (Paris) 90, 157–164 (1996).
Luscher, C., Nicoll, R. A., Malenka, R. C., & Muller, D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nature Neurosci. 3, 545–550 (2000).
Nicola, S. M., Surmeier, J. & Malenka, R. C. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu. Rev. Neurosci. 23, 185–215 (2000). A timely review of the physiological effects of dopamine on medium spiny neurons of the nucleus accumbens and dorsal striatum. It discusses dopamine modulation of ion channels through its actions on several subtypes of dopamine receptors. It also covers recent literature on the occurrence of long-term potentiation and long-term depression in these brain regions. Given the central role of dopamine-mediated transmission in drug reinforcement, this review provides a template within which the complex actions of drugs of abuse on the nucleus accumbens can be understood.
Jones, S., Kornblum, J. L. & Kauer, J. A. Amphetamine blocks long-term synaptic depression in the ventral tegmental area. J. Neurosci. 20, 5575–5580 (2000). Shows the development of long-term depression (LTD) at glutamate synapses in the ventral tegmental area (VTA). The authors show that amphetamine, by potentiating the actions of dopamine on D 2 -like receptors, completely abolishes LTD in the VTA. This effect could contribute to the demonstrated ability of amphetamine (and perhaps other drugs of abuse) to potentiate glutamate responses in the VTA (for example, see references 49,88 ) and possibly to cause sensitized behavioural responses as well.
Thomas, M. J., Malenka, R. C. & Bonci, A. Modulation of long-term depression by dopamine in the mesolimbic system. J. Neurosci. 20, 5581 –5586 (2000).This study, like reference 84 , shows that long-term depression (LTD) occurs at glutamate synapses in the ventral tegmental area (VTA), and that LTD is inhibited by dopamine acting at D 2 -like receptors. The authors also show LTD at glutamatergic synapses on medium spiny neurons of the nucleus accumbens, although no effect of dopamine on LTD was seen in this region.
Malinow, R., Mainen, Z. F., & Hayashi, Y. LTP mechanisms: from silence to four-lane traffic. Curr. Opin. Neurobiol. 10, 352–357 (2000).
Scannevin, R. H. & Huganir, R. L. Postsynaptic organization and regulation of excitatory synapses. Nature Rev. Neurosci. 1, 133–141 ( 2000).
Carlezon, W. A. Jr et al. Sensitization to morphine induced by viral-mediated gene transfer. Science 277, 812– 814 (1997).
Wolf, M. E. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog. Neurobiol. 54, 679– 720 (1998).
Bell, K., Duffy, P. & Kalivas, P. W. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology 23, 335–344 (2000).
Li, Y. et al. Both glutamate receptor antagonists and prefrontal cortex lesions prevent induction of cocaine sensitization and associated neuroadaptations . Synapse 34, 169–180 (1999).
Acknowledgements
This work was supported by grants from the National Institute on Drug Abuse.
Author information
Authors and Affiliations
Related links
Related links
DATABASE LINKS
Ca2+/calmodulin-dependent protein kinase IV
ENCYCLOPEDIA OF LIFE SCIENCES
Glossary
- LIMBIC SYSTEM
-
A collection of cortical and subcortical structures important for processing memory and emotional information. Prominent structures include the hippocampus and amygdala.
- CANNABINOIDS
-
Derivatives of 2-(2-2-isopropyl-5-methylphenyl)-5-pentyl-resorcinol, a molecule found in the plant Cannabis sativa. Cannabinoids are responsible for the psychoactive effects of marijuana.
- PHENCYCLIDINE
-
A potent psychoactive drug also known as angel dust, which has anaesthetic and analgesic actions. It blocks the NMDA receptor channel.
- MEDIUM SPINY NEURONS
-
The main cell population of the ventral and dorsal striatum; these GABA-mediated projection neurons form the two main outputs of these structures, called the direct and indirect pathways.
- TOLERANCE
-
Reduced drug responsiveness with repeated exposure to a constant drug dose.
- DEPENDENCE
-
Altered physiological state that develops to compensate for persistent drug exposure and that gives rise to a withdrawal syndrome after cessation of drug exposure.
- DYSPHORIA
-
Negative or aversive emotional state usually associated with anxiety and depression.
- SENSITIZATION
-
Enhanced drug responsiveness with repeated exposure to a constant dose.
- RAS PROTEINS
-
A group of small G proteins involved in growth, differentiation and cellular signalling that require the binding of GTP to enter into their active state.
- LOCUS COERULEUS
-
Nucleus of the brainstem. The main supplier of noradrenaline to the brain
- TYROSINE HYDROXYLASE
-
The rate-limiting enzyme in the biosynthesis of noradrenaline, dopamine and other catecholamines.
- WITHDRAWAL SYNDROME
-
A collection of signs and symptoms that appear after sudden cessation of drug intake. Depending on the drug, they can include mild shakiness, sweating, anxiety and even hallucinations.
- DOMINANT-NEGATIVE
-
A mutant molecule that forms heteromeric complexes with the wild type to yield a non-functional complex.
- ZINC FINGER
-
Protein module in which cysteine or cysteine–histidine residues coordinate a zinc ion. Zinc fingers are often used in DNA recognition and also in protein–protein interactions.
- GLUCOCORTICOIDS
-
Hormones produced by the adrenal cortex, which are involved in carbohydrate and protein metabolism, but also affect brain function. Cortisol (human) and corticosterone (rodent) are prime examples.
- PROTEASOME
-
Protein complex responsible for degrading intracellular proteins that have been tagged for destruction by the addition of ubiquitin.
- ARRESTINS
-
Inhibitory proteins that bind to phosphorylated receptors, blocking their interaction with G proteins and terminating signalling. For example, β-arrestin binds to phosphorylated β-adrenergic receptors and inhibits their ability to activate Gs.
- DYNAMIN
-
Protein involved in the formation of microtubule bundles and in membrane transport.
- CYCLIN-DEPENDENT KINASE 5
-
A member of a family of cyclin-dependent kinases, Cdk5 is enriched in brain, requires another protein termed p35 for its activation and is implicated in the regulation of neural growth and survival.
- LONG-TERM POTENTIATION
-
A long-lasting increase in the efficacy of synaptic transmission commonly elicited by high-frequency neuron stimulation.
Rights and permissions
About this article
Cite this article
Nestler, E. Molecular basis of long-term plasticity underlying addiction . Nat Rev Neurosci 2, 119–128 (2001). https://doi.org/10.1038/35053570
Published:
Issue Date:
DOI: https://doi.org/10.1038/35053570
This article is cited by
-
Effects of social housing conditions on ethanol-induced behavioral sensitization in Swiss mice
Psychopharmacology (2024)
-
Sex differences in mouse infralimbic cortex projections to the nucleus accumbens shell
Biology of Sex Differences (2023)
-
Whole-brain tracking of cocaine and sugar rewards processing
Translational Psychiatry (2023)
-
Sleep-mediated regulation of reward circuits: implications in substance use disorders
Neuropsychopharmacology (2023)
-
Hypoxia-inducible factor upregulation by roxadustat attenuates drug reward by altering brain iron homoeostasis
Signal Transduction and Targeted Therapy (2023)