Abstract
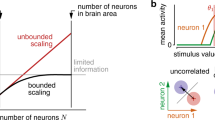
Neurons in the visual cortex are typically selective to a number of stimulus dimensions. Thus, there is a basic ambiguity in relating the response level of a single neuron to the stimulus values. It is shown that a multi-dimensional stimulus may be coded reliably by an ensemble of neurons, using a weighted average population coding model. Each neurons' contribution to the population signal for each dimension is the product of its response magnitude and its preferred value for that dimension. The sum of the products was normalized by the sum of the ensemble responses. Simulation results show that the representation accuracy increases as the square root of the number of units irrespective of the number of dimensions. Comparison of a specific 2D case of this population code for orientation and spatial frequency to behavioral discrimination levels yields that 103–104 neurons are needed to reach psychophysical performance. Introduction of each additional dimension requires about 1.7 times the number of neurons in the ensemble to reach the same level of accuracy. This result suggests that neurons may be selective for only 3 to 5 dimensions. It also provides another rationale for the existence of parallel processing streams in vision.
Similar content being viewed by others
References
Barlow HB (1972) Single units and sensation; a neuron doctrine for perceptual psychology? Perception 1:371–394
Barlow HB (1986) Why have multiple visual areas? Vision Res 26:81–90
Barlow HB, Foldiak P (1989) Adaptation and decorrelation in the cortex. In: Durbin R, Miall C, Mitchison G (eds) The computing neuron, chap 4. Addison Wesley, Worhengham, pp 54–72
Barlow HB, Kaushal TP, Hawken M, Parker AJ (1987) Human constrast discrimination and the threshold of cortical neurons. J Opt Soc Am A4: 2366–2371
Bradley A, Skottun BC (1984) The effects of large orientation and spatial frequency differences on spatial discriminations. Vision Res 24: 1889–1896
Bradley A, Skottun BC, Ohzawa I, Sclar G, Freeman RD (1987) Visual orientation and spatial frequency discrimination: a comparison of single neurons and behavior. J Neurophysiol 57: 755–772
Burbeck CA, Regan D (1983) Independence of orientation and size in spatial discriminations. J Opt Soc Am 73: 1691–1694
Desimone R, Schein SJ (1987) Visual properties of neurons in area V4 of the macaque: sensitivity to stimulus form. J Neurophysiol 57: 835–868
De Valois RL, De Valois KK (1988) Spatial vision. Oxford University Press, New York Oxford
De Valois RL, Albrecht DG, Thoreil LG (1982) Spatial frequency selectivity of cells in macaque visual cortex. Vision Res 22:545–559
DeYoe EA, Van Essen DC (1988) Concurrent processing streams in monkey visual cortex. Trends Neurosci 11:219–226
Felleman DJ, Van Essen DC (1987) Receptive field properties of neurons in area V3 of macaque monkey extrastriate cortex. J Neuorphysiol 57:889–920
Fuster JM (1990) Inferotemporal units in selective visual attention and short term memory. J Neuorphysiol 64:681–697
Geogopoulos AP, Schwartz AB, Kettner RE (1986) Neuronal population coding of movement direction. Science 233:1416–1419
Georgopoulos AP, Kettner RE, Schwartz AB (1988) Primate motor cortex and free arm movement to visual targets in three-dimensional space. II. Coding of direction of movement by a neuronal population. J Neurosci 8:2928–2937
Green DM, Swets JA (1974) Signal detection theory and psychophysics. Kreiger, New York
Lee C, Rohrer WH, Sparks DL (1988) Population coding of saccadic eye movements by neurons in the superior colliculus. Nature 332:357–360
Livingstone M, Hubel D (1988) Segregation of form, color, movement, and depth: anatomy, physiology and perception. Science 240:741–749
Miyashita Y, Chang HS (1988) Neuronal correlate of pictorial short term memory in primate temporal cortex. Nature 331:68–70
Newsome WT, Britten KH, Movshon JA (1989) Neural correlates of a perceptual decision. Nature 341:52–54
Orban GA (1984) Neuronal operations in the visual cortex. Springer, Berlin, Heidelberg New York
Sagi D (1988) The combination of spatial frequency and orientation is effortlessly perceived. Percept Psychophys 43:601–603
Sejnowsky TJ (1988) Neural populations revealed. Nature 332:308
Steinmetz MA, Motter BC, Duffy CJ, Mountcastle VB (1987) Functional properties of parietal visual neurons: Radial organization of directionalities with the visual field. J Neurosci 7:177–191
Treisman A, Gelade G (1980) A feature integration theory of attention. Cognitive Psychol 12:97–136
Van Essen DC (1985) Functional organization of primate visual cortex. In: Jones EG, Peters AA (eds) Cerebral cortex, vol 3. Plenum Press, New York 259–329
Vogels R (1990) Population coding of stimulus orientation by striate cortical cells. Biol Cybern 64:25–31
Vogels R, Orban GA (1990) How well do response changes of striate neurons signal differences in orientation: a study in the discriminating monkey. J Neuorsci 10:3543–3558
Vogels R, Spileers W, Orban GA (1989) The response variability of striate cortical neurons in the behaving monkey. Exp Brain Res 77:432–436
Watson AB (1983) Detection and recognition of simple spatial forms. In: Braddick OJ, Sleigh AC (eds) Physical and biological processing of images. Springer, Berlin Heidelberg New York, pp 100–114
Wilson HR (1983) Psychophysical evidence for spatial channels. In: Braddick OJ, Sleigh AC (eds) Physical and biological processing of images. Springer, Berlin Heidelberg New York, pp 88–99
Zohary E, Hillman P, Hochstein S (1990) Time course of perceptual discrimination and single neuron reliability. Biol Cybern 62:475–486
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zohary, E. Population coding of visual stimuli by cortical neurons tuned to more than one dimension. Biol. Cybern. 66, 265–272 (1992). https://doi.org/10.1007/BF00198480
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00198480