Abstract
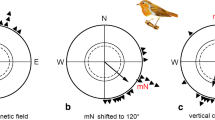
The orientation of phase-shifted control and hippocampal lesioned homing pigeons with previous homing experience was examined to investigate the possible participation of the hippocampal formation in sun compass orientation. Hippocampal lesioned pigeons displayed appropriate shifts in orientation indicating that such birds possess a functional sun compass that is used for orientation. However, their shift in orientation was consistently larger than in control pigeons revealing a difference in orientation never observed in pigeons that have not undergone a phase shift. Although alternative interpretations exist, the data suggest the intriguing possibility that following a change in the light-dark cycle, the hippocampal formation participates in the re-entrainment of a circadian rhythm that regulates sun compass orientation.
Similar content being viewed by others
References
Able KP (1991) Common themes and variations in animal orientation systems. Am Zool 31: 157–167
Batschelet E (1981) Circular statistics in biology. Academic Press, New York
Bingman VP, Hodos W (1992) Visual performance of homing pigeons following hippocampal lesion. Behav Brain Res 51: 203–209
Bingman VP, Jones T-J (1994) Sun compass-based spatial learning impaired in homing pigeons with hippocampal lesions. J Neurosci 14: 6687–6694
Bingman VP, Mench JA (1990) Homing behavior of hippocampus and parahippocampus lesioned pigeons following short-distance releases. Behav Brain Res 40: 227–238
Bingman VP, Yates G (1992) Hippocampal lesions impair navigational learning in experienced homing pigeons. Behav Neurosci 106: 229–232
Bingman VP, Bagnoli P, Ioalé P, Casini G (1984) Homing behavior in pigeons after telencephalic ablations. Brain Behav Evol 24: 94–106
Bingman VP, Ioalé P, Casini G, Bagnoli P (1988a) Unimpaired acquisition of spatial reference memory, but impaired homing performance in hippocampal ablated homing pigeons. Behav Brain Res 27: 179–188
Bingman VP, Ioalé P, Casini G, Bagnoli P (1988b) Hippocampal ablated homing pigeons show a persistent impairment in the time taken to return home. J Comp Physiol A 163: 559–563
Bingman VP, Ioalé P, Casini G, Bagnoli P (1990) The avian hippocampus: evidence for a role in the development of the homing pigeon navigational map. Behav Neurosci 104: 906–911
Bingman VP, Jones T-J, Strasser R, Gagliardo A, Ioalé P (1995) Homing pigeons, hippocampus and spatial cognition. In: Alleva E, Fasolo A, Lipp H-P, Nadel L, Ricceri L (eds) Behavioural brain research in naturalistic and semi-naturalistic settings. Kluwer, Dordrecht, pp 207–223
Chappell J, Guilford T (1995) Homing pigeons primarily use the sun compass rather than fixed directional visual cues in an open-field arena food-searching task. Proc R Soc Lond B Biol Sci 260: 59–63
Eichenbaum H, Stewart C, Morris RGM (1990) Hippocampal representation in place learning. J Neurosci 10: 3531–3542
Foá A, Saviozzi G (1990) Effects of melatonin on sun-compass orientation of homing pigeons. J Biol Rhythms 5: 17–24
Gagliardo A, Mazzotto M, Bingman VP (1996) Hippocampal lesion effects on learning strategies in homing pigeons. Proc R Soc Lond B Biol Sci 263: 529–534
Ioalé P, Nozzolini M, Papi F (1990) Homing pigeons do extract directional information from olfactory stimuli. Behav Ecol Sociobiol 26: 301–305
Jacobs LF, Gaulin SJC, Sherry DF, Hoffman GE (1990) Evolution of spatial cognition: sex-specific patterns of spatial behavior predict hippocampal size. Proc Natl Acad Sci USA 87: 6349–6352
Jarrard LE (1993) On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol 60: 9–26
Kramer G (1959) Recent experiments on bird orientation. Ibis 101: 399–416
Kramer G, Riese E (1952) Die Dressur von Brieftauben auf Kompassrichtung in Wahlkaefig. Z Tierpsychol 9: 245–251
Krebs JR, Sherry DF, Healy SD, Perry VH, Vaccarino AL (1989) Hippocampal specialization of food-storing birds. Proc Natl Acad Sci USA 86: 1388–1392
Krushinskaya N (1966) Some complex forms of feeding behaviour of nutcracker Nucifraga caryocatactes, after removal of old cortex. Zh Evol Biokhim Fiziol 2: 563–568
McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs WE, Suster M, Weaver KL (1996) Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J Exp Biol 199: 173–185
Meck WH, Church RM, Olton DS (1984) Hippocampus, time and memory. Behav Neurosci 98: 3–22
Morris RGM, Garrud P, Rawlins J, O'Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297: 681–683
Nadel L (1991) The hippocampus and space revisited. Hippocampus 1: 221–229
O'Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map: preliminary evidence from unit activity in the freely moving rat. Brain Res 34: 171–175
O'Keefe J, Nadel L (1978) The hippocampus as a cognitive map. Clarendon Press, Oxford
Olton DS, Becker J, Handelmann G (1979) Hippocampus, space and memory. Behav Brain Sci 2: 313–365
Papi F, Maffei L, Giongo F (1985) Pineal body and bird navigation: new experiments on pinealectomized pigeons. Z Tierpsychol 67: 257–268
Rehkämper G, Haase E, Frahm HD (1988) Allometric comparison of brain weight and brain structure volumes in different breeds of domestic pigeons, Columba livia f.d. (fantails, homing pigeons, strassers). Brain Behav Evol 37: 85–91.
Schmidt-Koenig K (1979) Avian orientation and navigation. Academic Press, New York
Schwegler H, Crusio WE, Lipp H-P, Heimrich B (1988) Water-maze learning in the mouse correlates with variation in hippocampal morphology. Behav Genet 18: 153–165
Sherry DF, Vaccarino AL (1989) The hippocampus and memory for food caches in black-capped chickadees. Behav Neurosci 103: 308–318
Sherry DF, Vaccarino AL, Buckenham K, Herz RS (1989) The hippocampal complex of food-storing birds. Brain Behav Evol 34: 308–317
Sherry DF, Forbes MRL, Khurgel M, Ivy GO (1993) Females have a larger hippocampus than males in the broodparasitic brown-headed cowbird. Proc Natl Acad Sci USA 90: 7839–7843
Smale L, Morin LP (1990) Photoperiodic responsiveness of hamsters with lesions of the lateral geniculate nucleus is related to hippocampal damage. Brain Res Bull 24: 185–190
Stankov B, Fraschini F, Russel RJ (1993) The melatonin receptor: distribution, biochemistry, and pharmacology. In: Yu H-S, Reiter RG (eds) Melatonin biosynthesis, physiological effects and clinical applications. CRC Press, Boca Raton, Fla, pp 155–186
Taube JS, Muller RU, Ranck JB Jr (1990) Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10: 420–435
Wallraff H (1974) Das Navigationssystem der Voegel. Oldenbourg, Munich
Wallraff HG, Kiepenheuer J (1994) Relationships between initial orientation and subsequent homing of individual pigeons. Anim Behav 47: 821–832
Wiltschko R, Kumpfmüller R, Muth R, Wiltschko W (1994) Pigeon homing: the effect of a clock-shift is often smaller than predicted. Behav Ecol Sociobiol 35: 63–73
Wiltschko W, Balda RP (1989) Sun compass orientation in seedcaching scrub jays (Aphelocoma coerulescens) J Comp Physiol A 164: 717–721
Wiltschko W, Wiltschko R (1987) Cognitive maps and navigation in homing pigeons. In: Ellen P, Thinus-Blanc C (eds) Cognitive processes and spatial orientation in animal and man. Nijhoff, Dordrecht, pp 201–216
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bingman, V.P., Gagliardo, A. & Ioalé, P. Hippocampal participation in the sun compass orientation of phase-shifted homing pigeons. J Comp Physiol A 179, 695–702 (1996). https://doi.org/10.1007/BF00216133
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00216133