Summary
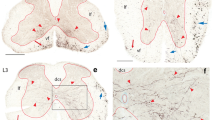
Primary afferent projections from cervical and lumbar levels were studied in the turtle Pseudemys scripta elegans. Injections of radioactive amino acids, wheat germ agglutinin and horseradish peroxidase were made into the dorsal root ganglia or the spinal cord. Previous reports on the terminal distribution of primary afferents within the ipsilateral segment of entry were confirmed (Kusuma and ten Donkelaar 1979, 1980) and additional dorsal root projections were demonstrated to the contralateral side and to several neighboring spinal segments. The primary afferent projections to the brainstem were essentially restricted to a dorsolateral area that appears to be homologous to the main dorsal column nuclei (n. gracilis and n. cuneatus medialis) in mammals. While exhibiting a similarly extensive rostro-caudal span, the projections originating from lumbar injections terminated more medially, those from cervical injections more laterally. The labeling pattern suggested that terminations are mainly on dorsally extending dendrites.
Similar content being viewed by others
References
Albright BC, Haines DE (1978) Dorsal column nuclei in a prosimian primate (Galago senegalensis). II. Cuneate and lateral cuneate nuclei: Morphology and primary afferent fibers from cervical and upper thoracic spinal segments. Brain Behav Evol 15:165–184
Antal M, Tornai I, Székely G (1980) Longitudinal extent of dorsal root fibers in the spinal cord and brain stem of the frog. Neurosci 5:1311–1322
Basbaum AI, Hand PJ (1973) Projections of cervico-thoracic dorsal roots to the cuneate nucleus of the rat, with observations on cellular “bricks”. J Comp Neurol 148:347–360
Beck CHM (1981) Mapping of forelimb afferents to the cuneate nuclei of the rat. Brain Res Bull 6:503–516
Beckstead RM, Norgren R, (1979) An autoradiographic examination of the central distribution of the trigeminal, facial, glossopharyngeal and vagal nerves in the monkey. J Comp Neurol 184:455–472
Berkley KJ, Hand PJ (1978) Efferent projections of the gracile nucleus in the cat. Brain Res 153:263–284
Berkley KJ, Worden IG (1978) Projections to the inferior olive of the cat. I. Comparisons of input from the dorsal column nuclei, the lateral cervical nucleus, the spino-olivary pathways, the cerebral cortex and the cerebellum. J Comp Neurol 180:237–252
Boivie J (1978) Anatomical observations on the dorsal column nuclei, their thalamic projection and cytoarchitecture of some somatosensory thalamic nuclei in the monkey. J Comp Neurol 178:17–48
Burton H, Loewy AD (1977) Projections to the spinal cord from medullary somatosensory relay nuclei. J Comp Neurol 173:773–792
Carpenter MB, Stein BM, Shriver JE (1968) Central projections of the spinal dorsal roots in the monkey. II. Lower thoracic, lumbosacral and coccygeal dorsal roots. Am J Anat 123:75–118
Cohen DH, Karten HJ (1974) The structural organization of the avian brain: A overview. In: Goodman IJ, Schein MW (eds) Birds: Brain and behavior, Academic, New York, San Francisco, London pp 29–73
Cruce WLR, Nieuwenhuys R (1974) The cell masses in the brain stem of the turtle Testudo hermanni; a topographical and topological analysis. J Comp Neurol 156:277–306
Culberson JL, Kimmel DL (1975) Primary afferent fiber distribution at brachial and lumbosacral spinal cord levels in the opossum (Didelphis marsupialis virginiana). Brain Behav Evol 12:229–246
Culberson JL, Haines DE, Kimmel DL, Brown PB (1979) Contralateral projection of primary afferent fibers to mammalian spinal cord. Experimental Neur 64:83–97
Donkelaar HJ ten, Nieuwenhuys R (1979) The brain stem of reptiles. In: Gans C et al. Biology of the reptilia, Academic London, New York pp 133–200
Donkelaar HJ ten, Kusuma A, de Boer-van Huizen R (1980) Cells of origin of pathways descending to the spinal cord in some quadrupedal reptiles. J Comp Neurol 192:827–851
Ebbesson SOE (1978) Somatosensory pathways in lizards: The identification of the medial lemniscus and related structures. In: Greenberg N, Mac Lean PD (eds), NIMH, Rockville Md pp 91–104
Edwards SB (1972) The ascending and descending projections of the red nucleus in the cat: an experimental study using an autoradiographic tracing method. Brain Res 48:45–63
Ellis LC, Rustioni A (1981) A correlative HRP, Golgi and EM study of the intrinsic organization of the feline dorsal column nuclei. J Comp Neurol 197:341–367
Feldman SG, Kruger L (1980) An axonal transport study of the ascending projection of medial lemniscal neurons in the rat. J Comp Neurol 192 (1980) 427–454
Finger TE (1978) Cerbellar afferents in teleost cat fish (Ictaluridae). J Comp Neurol 181:173–182
Glees P, Soler J (1951) Fibre content of the posterior column and synaptic connections of nucleus gracilis. Z Zellforsch 36:381–400
Goldby F, Robinson LR (1962) The central connections of dorsal spinal nerve roots and the ascending tracts in the spinal cord of Lacerta viridis. J Anat 96:153–170
Gray TS, Hazlett JC, Martin GF (1981) Organization of projection from the gracile, medial cuneate and lateral cuneate nuclei in the North American Opossum. Horseradish peroxidase study of the cells projecting to the cerebellum, thalamus and spinal cord. Brain Behav Evol 18:140–156
Groenewegen HJ, Boesten AJP, Voogd J (1975) The dorsal column nuclear projections to the nucleus ventralis posterior lateralis thalami and the inferior olive in the cat. An autoradiographic study. J Comp Neurol 162:505–518
Gulley RL (1973) Golgi studies of the nucleus gracilis in the rat. Anat Rec 177:325–342
Hand PJ (1966) Lumbosacral dorsal root terminations in the nucleus gracilis of the cat. Some observations on the terminal degeneration in other medullary sensory nuclei. J Comp Neurol 126:137–156
Hazlett JC, Dom R, Martin GF (1972) Spino-bulbar, spino-thalamic and medial lemniscal connections in the American opossum, Didelphis marsupialis virginiana. J Comp Neurol 146:95–118
Holbrook JR, Wilcox H (1964) Observations on the spino-cerebellar tracts following dorsal root transsection in the goat. Anat Rec 148:292
Imai Y, Kusama T (1969) Distribution of the dorsal root fibers in the cat. An experimental study with the Nauta method. Brain Res 13:338–359
Jacobs VL, Sis RF (1980) Ascending projections of the dorsal column in a garter snake (Thamnophis siritalis): A degeneration study. Anat Rec 196:37–50
Joseph BS, Whitlock DG (1968a) Central projections of selected spinal dorsal root in anuran amphibians. Anat Rec 160:279–288
Joseph BS, Whitlock DG (1968b) Central projections of brachial and lumbar dorsal roots in reptiles. J Comp Neurol 132:469–484
Joseph BS, Whitlock DG (1968c) The morphology of spinal afferent-efferent relationships in vertebrates. Brain Behav Evol 1:2–18
Kalil K (1979) Projections of the cerebellar and dorsal column nuclei upon the inferior olive in the rhesus monkey. An autoradiographic study. J Comp Neurol 188:43–63
Karten HJ, Konishi M, Pettigrew J (1978) Somatosensory representation in the anterior wulst of the owl (Speotyto cunicularia). Soc Neurosci Abstr 4:554
Keller JH, Hand PJ (1970) Dorsal root projections to nucleus cuneatus of the cat. Brain Res 20:1–17
Künzle H (1982) Dorsal root projections to the cerebellum in turtle. Exp Brain Res 45:464–466
Künzle H (1983) Supraspinal cell populations projecting to the cerebellar cortex in the turtle, Pseudemys scripta elegans. Exp Brain Res (in press)
Künzle H, Woodson W (1981) Dorsal root projections in turtle. Neurosci Lett Suppl 7:136
Künzle H, Woodson W (1982) Meso-diencephalic and other target regions of ascending spinal projections in the turtle, Pseudemys scripta elegans. J Comp Neurol (in press)
Kusuma A, ten Donkelaar HJ (1979) Staining of the dorsal root primary afferent fibers by anterograde movement of horseradish peroxidase and retrograde labeling of motoneurons and preganglionic autonomic cells in the turtle spinal cord. Neurosci Lett 14:141–146
Kusuma A, ten Donkelaar HJ (1980) Dorsal root projections in various types of reptiles. Brain Behav Evol 17:291–309
Kuypers HGJM, Tuerk JD (1964) The distribution of the cortical fibers within the nuclei cuneatus and gracilis in the cat. J Anat 98:143–162
Leonard RB, Cohen PH (1975) Spinal terminal fields of dorsal root fibers in the pigeon (Columba livia). J Comp Neurol 163:181–192
Liu CN (1956) Afferent nerves to Clarke's and the lateral cuneate nuclei in the cat. Arch Neurol 75:66–77
Martin GF, Dom R, Katz S, King J (1974) The organization of projection neurons in the opossum red nucleus. Brain Res 78:17–34
Mesulam MM (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: A non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26:106–117
Neary TJ, Wilczynski W (1977) Ascending thalamic projections from the obex region in ranid frogs. Brain Res 138:529–533
Odutola AB (1977) On the location of reticular neurons projecting to the cuneo-gracile nuclei in the cat. Exp Neurol 54:54–59
Rustioni A, Kaufman AB (1977) Identification of cells of origin of non-primary afferents to the dorsal column nuclei of the cat. Exp Brain Res 27:1–14
Rustioni A, Macchi G (1968) Distribution of dorsal root fibers in the medulla oblongata of the cat. J Comp Neurol 134:113–126
Salibi NA, Saadé NE, Banna NR, Jabbur SJ (1980) Dorsal column input into the reticular formation. Nature 288:481–483
Schroeder DM, Jane JA (1976) The intercollicular area of the inferior colliculus. Brain Behav Evol 13:125–141
Schwab ME (1979) Variation in the rhombencephalon. In: Gans C et al (eds) Biology of the reptilia Vol 9, Academic Press, London, New York, San Francisco pp 201–246
Schwab ME, Javoy-Agid F, Agid Y (1978) Labeled wheat germ agglutinin (WGA) as a new, highly sensitive retrograde tracer in the rat brain hippocampal system. Brain Res 152:145–150
Shriver JE, Stein BM, Carpenter MB (1968) Central projections of spinal dorsal roots in the monkey. I. Cervical and upper thoracic dorsal roots. Am J Anat 123:27–74
Somana R, Walberg F (1980) A re-examination of the cerebellar projections from the gracile, main and external cuneate nuclei in the cat. Brain Res 186:33–42
Sotigu ML, Marini G (1977) Reticulo-cuneate projections as revealed by horseradish peroxidase axonal transport. Brain Res 128:341–345
Sterling P, Kuypers HGJM (1967) Anatomical organization of the brachial spinal cord of the cat. I. The distribution of dorsal root fibers. Brain Res 4:1–15
Székely G (1976) The morphology of motoneurons and dorsal root fibers in the frog's spinal cord. Brain Res 103:275–290
Valverde F (1966) The pyramidal tract in rodents. A study of its relations with the posterior column nuclei, dorsolateral reticular formation of the medulla oblongata and cervical spinal cord (Golgi and electron microscopic observations). Z Zellforsch mikr Anat 71:297–363
Walberg F (1966) The fine structure of the cuneate nucleus in normal cats and following interruption of afferent fibers; an electron microscopical study with particular reference to findings made in Glees and Nauta sections. Exp Brain Res 2:107–128
Weisberg JA, Rustioni A (1979) Differential projections of cortical sensorimotor areas upon dorsal column nuclei of cats. J Comp Neurol 184:401–422
Wen CY, Tan CK, Wong WC (1979) Experimental degeneration of primary afferent terminals in the cuneate nucleus of the monkey (Macacus fascicularis). J Anat 128:709–720
Woodson W, Künzle H (1982) Distribution and structural characterization of neurons giving rise to descending spinal projections in the turtle, Pseudemys scripta elegans. J Comp Neurol (in press)
Zeehandelaar I (1921) Ontogenese und Phylogenese der Hinterstrangkerne in Verband mit der Sensibilität. Fol Neurobiol 12:1–133
Zeier H, Karten HJ (1971) The archistriatum of the pigeon: Organization of afferent and efferent connections. Brain Res 31:313–326
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Künzle, H., Woodson, W. Primary afferent projections to the spinal cord and the dorsal column nuclear complex in the turtle Pseudemys. Anat Embryol 166, 229–245 (1983). https://doi.org/10.1007/BF00305085
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00305085