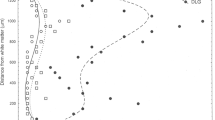
Summary
The laminar distribution of neuron losses in posterior cingulate cortex were evaluated in 25 clinically and neuropathologically diagnosed cases of dementia of the Alzheimer type (DAT). The layer of maximal neuron loss in area 23a for each DAT case was determined by comparison with mean neuron densities for each layer of 17 neurologically intact control cases. The DAT cases were separated into five classes: class 1, 12% of all DAT cases, no or less than 40% neuron loss in any layer; class 2, 24%, maximal neuron losses in layers II or III; class 3, 28%, losses mainly in layer IV; class 4, 12%, losses mainly in layers V or VI; class 5, 24%, severe losses in all layers. An analysis of large and small neurons showed that in class 2 there was an equal loss of both in layer IIIa−b, in class 3 mostly small neurons were lost in layer IV, in class 4 mostly large neurons were lost in layers III, IV and V, while in class 5 there was no selectivity. The age of disease onset and length of the disease were the same for all classes, although classes 4 and 5 tended to have an earlier onset. No measures of thioflavin S-stained neuritic plaque (NP) or neurofibrillary tangle (NFT) density discriminated among these classes. In 64% of all DAT cases there was a progressive shift in NFT from ventral area 30 where most were in layer II to areas 23a−b where there was a balance between those in superficial and deep layers to dorsal area 23c where most were in layers V and VI. There were four cases with massive numbers of NFT in area 23a (1802±477 versus 261±44 for all other cases). Since one of these cases was in each of classes 1, 2, 3 and 5, it is unlikely that this form of amyloid deposition is related to laminar specificities in neuron degeneration. Finally, neuron and NFT densities in the hippocampal formation were the same for each class. In conclusion, differential laminar changes in neuron density provide a basis for neuropathological subtyping of DAT which is more sensitive than measures of thioflavin S-stained NP and NFT densities either in neocortex or the hippocampus.
Similar content being viewed by others
References
Baleydier C, Mauguiere F (1980) The duality of the cingulate gyrus in monkey. Neuroanatomical study and functional hypothesis. Brain 103:525–554
Ball MJ (1977) Neuronal loss, neurofibrillary tangles and granulovacuolar degeneration in the hippocampus with ageing and dementia. Acta Neuropathol (Berl) 37:111–118
Beal MF, Mazurek MF, Tran VT, Chattha G, Bird ED, Martin JB (1985) Reduced numbers of somatostatin receptors in the cerebral cortex in Alzheimer's disease. Science 229:289–291
Bird TD, Stranahan S, Sumi SM, Raskin M (1983) Alzheimer's disease: choline acetyltransferase activity in brain tissue from clinical and pathological subgroups. Ann Neurol 14:284–293
Braak H (1979) Pigment architecture of the human telencephalic cortex IV. Regio retrosplenialis. Cell Tissue Res 204:431–440
Braak H, Braak E (1986) Ratio of pyramidal cells versus nonpyramidal cells in the human frontal isocortex and changes in ratio with ageing and Alzheimer's disease. Prog Brain Res 40:185–212
Braak H, Braak E, Kalus P (1989) Alzheimer's disease: areal and laminar pathology in the occipital isocortex. Acta Neuropathol 77:494–506
Brun A, Englund E (1981) Regional pattern of degeneration in Alzheimer's disease: neuronal loss and histopathological grading. Histopathology 5:549–564
Chan-Palay V, Lang W, Allen YS, Haesler U, Polak JM (1985) II. Cortical neurons immunoreactive with antisera against neuropeptide Y are altered in Alzheimer's-type dementia. J Comp Neurol 238:390–400
Chui HC, Teng EL, Henderson VW, Moy AC (1985) Clinical subtypes of dementia of the Alzheimer type. Neurology 35:1544–1550
Couch JV (1982) Fundamentals of statistics for the behavioral sciences, St Martin's Press, New York, pp 266–270
Davies P, Katzman R, Terry RD (1980) Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer disease and Alzheimer senile dementia. Nature 288:279–280
Duffy P, Mayeux R, Kupsky W (1988) Familial Alzheimer's disease with myoclonus and ‘spongy change’. Arch Neurol 45:1097–1100
Ebinger G, Bruyland M, Martin JJ, Herregodts P, Cras P, Michotte Y, Gomme L (1987) Distribution of biogenic amines and their catabolites in brains from patients with Alzheimer's disease. J Neurol Sci 77:267–283
Ferrier IN, Crass AJ, Johnson JA, Roberts GW, Crow TJ, Corsellis JAN, Lee YC, O'Shaughnessy D, Adrian TE, McGreger GP, Baracese-Hamilton AJ, Bloom SR (1983) Neuropeptides in Alzheimer type dementia. J Neurol Sci 62:159–170
Gabriel M, Foster K, Orona E, Saltwick SE, Stanton M (1980) Neuronal activity of cingulate cortex, anteroventral thalamus, and hippocampal formation in discriminative conditioning: encoding and extraction of the significance of conditional stimuli. Prog Psychobiol Physiol Psychol 9:125–231
Hof PR, Bouras C, Constantinidis J, Morrison JH (1989) Balint's syndrome in Alzheimer's disease: specific disruption of the occipito-parietal visual pathway. Brain Res 493:368–375
Hyman BT, Van Hoesen GW, Kromer LJ, Damasio AR (1986) Perforant pathway changes and the memory impairment of Alzheimer's disease. Ann Neurol 20:472–481
Hyman BT, Kromer LJ, Van Hoesen GW (1988) A direct demonstration of the perforant pathway terminal zone in Alzheimer's disease using the monoclonal antibody Alz-50. Brain Res 450:392–397
Kaitz SS, Robertson RT (1981) Thalamic connections with limbic cortex II. Corticothalamic projections. J Comp Neurol 195:527–545
Kaye JA, May C, Attack JR, Daly E, Sweeney DL, Beal MF, Kaufman S, Milstein S, Friedland RP, Rapoport SI (1988) Cerebrospinal fluid neurochemistry in the myoclonic subtype of Alzheimer's disease. Ann Neurol 24:647–650
Lewis DA, Campbell MJ, Terry RD, Morrison JH (1987) Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer's disease: a quantitative study of visual and auditory cortices. J Neurosci 7:1799–1808
Lubar JF (1964) Effect of medial cortical lesions on the avoidance behavior of the cat. J Comp Physiol Phychol 58:38–46
Lubar JF, Perachio AA (1965) One-way and two-way learning and transfer of an active avoidance response in normal and cingulectomized cats. J Comp Physiol Phychol 60:46–52
Mann DMA, Yates PO; Marcyniuk B (1985) Some morphometric observations on the cerebral cortex and hippocampus in presenile Alzheimer's disease, senile dementia of the Alzheimer type and Down's syndrome in middle age. J Neurol Sci 69:139–159
Mantyh PW (1982) Forebrain projections to the periaqueductal gray in the monkey, with observations in cat and rat. J Comp Neurol 206: 146–158
Matsunami K, Kawashima T, Satake H (1989) Mode of [14C]2-deoxy-d-glucose uptake into retrosplenial cortex and other memory-related structures of the monkey during a delayed response. Brain Res Bull 22:829–838
Mayeux R, Stern Y, Spanton S (1985) Heterogeneity in dementia of the Alzheimer type: evidence of subtypes. Neurology 35:453–461
Mesulam M-M, Van Hoesen GW, Pandya DN, Geschwind N (1977) Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: a study with a new method for horseradish peroxidase histochemistry. Brain Res 136:393–414
Morecraft RJ, Van Hoesen GW (1988) Somatotopical organization of cingulate projections to the primary and supplementary motor cortices in the old-world monkey. Neurosci Abstr 14:820
Morrell JI, Greenberger LM, Pfaff DW (1981) Hypothalamic, other diencephalic, and telencephalic neurons that project to the dorsal midbrain. J Comp Neurol 201:589–620
Morrison JH, Rogers J, Scherr S, Benoit R, Bloom FE (1985) Somatostatin immunoreactivity in neuritic plaques of Alzheimer's patients. Nature 314:90–92
Mountjoy CQ, Roth M, Evans NJR, Evans HM (1983) Cortical neuronal counts in normal elderly controls and demented patients. Neurobiol Aging 4:1–11
Neary D, Snowdon JS, Mann DMA, Bowen DM, Sims NR, Northern B, Yates PO; Davison AN (1986) Alzheimer's disease: a correlative study. J Neurol Neurosurg Psychiatry 49:229–237
Pearson RCA, Esiri MM, Hiorns RW, Wilcock GK, Powell TPS (1985) Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci USA 82:4531–4534
Roberts GW, Crow TJ, Polak JM (1985) Location of neuronal tangles in somatostatin neurones in Alzheimer's disease. Nature 314:92–94
Rogers J, Morrison JH (1985) Quantitative morphology and regional and laminar distributions of senile plaques in Alzheimer's disease. J Neurosci 5:2801–2808
Rossor MN, Emson PC, Mountjoy CQ, Roth M, Iversen LL (1980) Reduced amounts of immunoreactive somatostatin in the temporal cortex in senile dementia of Alzheimer type. Neurosci Lett 20:373–377
Rossor MN, Iversen LL, Reynolds GP, Mountjoy CQ, Roth M (1984) Neurochemical characteristics of early and late onset types of Alzheimer's disease. Brit. Med. J 288:961–964
Royce GJ (1982) Laminar origin of cortical neurons which project upon the caudate nucleus: a horseradish peroxidase investigation in the cat. J Comp Neurol 205:8–29
Royce GJ (1983) Cells of origin of corticothalamic projection upon the centromedian and parafascicular nuclei in the cat. Brain Res 258:11–21
Schwartz PH, Kurucz J, Kurucz A (1964) Recent observations on senile cerebral changes and their pathogenesis. J Am Geriat Soc 12:908–922
Seltzer B, Sherwin I (1983) A comparison of clinical features in early and late onset primary degenerative dementia one entity or two? Arch Neurol 40:143–146
Sutherland RJ, Whishaw IQ, Kolb B (1988) Contributions of cingulate cortex to two forms of spatial learning and memory. J Neurosci 8:1863–1872
Terry RD, Peck A, DeTeresa R, Schechter R, Horoupian S (1981) Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol 10:184–192
Terry RD, Hansen LA, DeTeresa R, Davies P, Tobias H, Katzman R (1987) Senile dementia of the Alzheimer type without neocortical neurofibrillary tangles. J Neuropathol Exp Neurol 46:262–268
Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT (1987) Retrosplenial amnesia. Brain 110:1631–1646
Vilensky JA, Van Hoesen GW (1981) Corticopontine projections from the cingulate cortex in the rhesus monkey. Brain Res 205:391–395
Vogt BA (1976) Retrosplenial cortex in the rhesus monkey: a cytoarchitectonic and Golgi study. J Comp Neurol 169:63–98
Vogt BA (1985) Cingulate cortex. In: Peters A, Jones EG (eds) Cerebral Cortex, vol 4. Plenum Publishing, New York, pp 89–149
Vogt BA, Pandya DN (1987) Cingulate cortex of the rhesus monkey II. Cortical afferents. J Comp Neurol 262:271–289
Vogt BA, Pandya DN, Rosene DL (1987) Cingulate cortex of the rhesus monkey I. Cytoarchitecture and thalamic afferents. J Comp Neurol 262:256–270
Watson RT, Heilman KM, Cauthen JC, King FA (1973) Neglect after cingulectomy. Neurology 23:1003–1007
Yeterian EH, Van Hoesen GW (1978) Cortico-striate projections in the rhesus monkey: the organization of certain corticocaudate connections. Brain Res 139:43–63
Zilles K, Armstrong E, Schlaug G, Schleicher A (1986) Quantitative cytoarchitectonics of the posterior cingulate cortex in primates. J Comp Neurol 253:514–524
Author information
Authors and Affiliations
Additional information
Supported by NIH-NINDS grants, NS18745, NS14944 and PONS19632
Rights and permissions
About this article
Cite this article
Vogt, B.A., Van Hoesen, G.W. & Vogt, L.J. Laminar distribution of neuron degeneration in posterior cingulate cortex in Alzheimer's disease. Acta Neuropathol 80, 581–589 (1990). https://doi.org/10.1007/BF00307624
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00307624