Summary
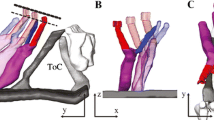
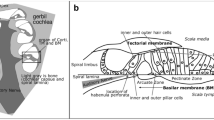
The lateral membrane system of the cochlear outer hair cell, consisting of the lateral plasma membrane, pillars, filamentous lattice and subsurface cisternae, is considered to be involved in the contractile movement of the isolated cochlear outer hair cell. The filamentous lattice, called the cytoskeletal spring, has been identified in the demembranated cochlear outer hair cell treated with the detergent Triton X-100. In this study, the quick-freeze, deep-etch method was applied to demonstrate the three-dimensional organization of both the filamentous and membranous structures of the lateral membrane system of cochlear outer hair cells. Treatment with saponin revealed that the inner leaflet of the lateral plasma membrane of the cochlear outer hair cell possesses more membrane particles than the outer leaflets, and that the pillars are closely associated with membrane particles in the inner leaflet of the lateral membrane. The presence of filamentous bridges between the filamentous lattice and the subsurface cisternae was also detected. We propose that the lateral membrane system in the cochlear outer hair cell may play an important role in the tuning mechanisms within the cochlea in normal hearing.
Similar content being viewed by others
References
Arima T, Shibata Y, Yamamoto T (1985) Three-dimensional visualization of basal body structures and some cytoskeletal components in the apical zone of tracheal ciliated cells. J Ultrastruct Res 93:61–70
Arima T, Uemura T, Yamamoto T (1986) Cytoskeletal organization in the supporting cell of the guinea pig organ of Corti. Hear Res 24:169–175
Arima T, Uemura T, Yamamoto T (1987) Three-dimensional visualizations of the inner ear hair cell of the guinea pig. A rapidfreeze, deep-etch study of filamentous and membranous organelles. Hear Res 25:61–68
Ashmore JF (1987) A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol (Lond) 388:323–347
Brownell WE, Bader CR, Bertrand D, Ribaupierre YD (1985) Evoked mechanical responses of isolated cochlear outer hair cell. Science 227:194–196
Drenckhahn D, Kellner J, Mannherz HG, Groschel-Stewart U, Kendrick-Jones J, Scholey J (1982) Absence of myosin-like immunoreactivity in stereocilia of cochlear hair cells. Nature 300:531–532
Engstrom H, Sjostrand FS (1954) The structure and innervation of the cochlear hair cells. Acta Otolaryngol 44:490–501
Ferguson DG, Schwartz HW, Frantini-Armstrong C (1984) Subunit structure of junctional feet in triads of skeletal muscle: a freeze-drying, rotary-shadowing study. J Cell Biol 99:1735–1742
Flock A, Cheung HC (1977) Actin filaments in sensory hairs of inner ear receptor cell. J Cell Biol 75:339–343
Flock A, Bretscher A, Weber K (1982) Immunohistochemical localization of several cytoskeletal proteins in inner ear sensory and supporting cells. Hear Res 6:75–89
Flock A, Flock B, Ulfendahl M (1986) Mechanisms of movement in outer hair cells and possible structural basis. Arch Otorhinolaryngol 243:83–90
Franzini-Armstrong C, Nunzi G (1983) Junctional feet and particles in the triads of a fast-twitch muscle fibre. J Muscle Res Cell Motil 4:233–252
Franzini-Armstrong C, Kenny LJ, Varriano-Marton E (1987) The structure of calsequestrin in triads of vertebrate skeletal muscle; a deep-etch study. J Cell Biol 105:49–56
Gulley RL, Reese TS (1977) Regional specialization of the hair cell plasmalemma in the organ of Corti. Anat Rec 189:109–124
Heuser JE, Salpeter SR (1979) Organization of acetylcholine receptors in quick-frozen, deep-etch and rotary-replicated Torpedo postsynaptic membrane. J Cell Biol 82:150–173
Holley MC, Ashmore JF (1988a) On the mechanism of a high-frequency force generator in outer hair cells isolated from the guinea pig cochlea. Proc R Soc Lond [Biol] 232:413–429
Holley MC, Ashmore JF (1988b) A cytoskeletal spring in cochlear outer hair cells. Nature 335:635–637
Holley MC, Ashmore JF (1990) A cytoskeletal spring for the control of cell shape in outer hair cells isolated from the guinea pig cochlea. Eur Arch Otorhinolaryngol 247:4–7
Jorgensen AO, Shen ACY, Cambell KP, Maclennan DH (1983) Ultrastructural localization of calsequestrin in rat skeletal muscle by immunoferritin labeling, of ultrathin frozen sections. J Cell Biol 97:1573–1581
Kachar B, Brownell WE, Altschuler R, Fex J (1986) Electrokinetic shape changes of cochlear outer hair cells. Nature 322:365–368
Kimura RS (1975) The ultrastructure of the organ of Corti. Int Rev Cytol 42:173–222
Lim DJ, Hanamure Y, Ohashi Y (1989) Structural organization of the outer hair cell wall. Acta Otolaryngol 107:398–405
Macartney JC, Comis SD, Pickles JO (1980) Is myosin in the cochlea a basis for active motility. Nature 288:491–492
Prieto J, Merchan JA, Gil-Loyzaga P, Rueda J (1986) Subsurface material in outer hair cells. Hear Res 21:277–280
Rabie A, Thomasset M, Legrand Ch (1983) Immunocytochemical detection of calcium-binding protein in the cochlear and vestibular hair cells of the rat. Cell Tissue Res 232:691–696
Raphael Y, Wroblewski R (1986) Linkage of sub-membranecisterns with the cytoskeleton and the plasma membrane in cochlear outer hair cells. J Submicrosc Cytol 18:731–737
Saito K (1983) Fine structure of the sensory epithelium of guinea-pig organ of Corti: subsurface cisternae and lamellar bodies in the outer hair cells. Cell Tissue Res 229:467–481
Shibata Y, Arima T, Yamamoto T (1983) Double-axis rotary replication for deep-etching. J Microsc 136:121–123
Slepecky N, Chamberlain SC (1985) Immunoelectron microscopic and immunofluorescent localization of cytoskeletal and muscle-like contractile proteins in inner ear sensory hair cells. Hear Res 20:245–260
Slepecky N, Ulfendahl M, Flock A (1988a) Effects of caffeine and tetracaine on outer hair cell shortening suggest intracellular calcium involvement. Hear Res 32:11–22
Slepecky N, Ulfendahl M, Flock A (1988b) Shortening and elongation of isolated outer hair cells in response to application of potassium gluconate, acetylcholine and cationized ferritin. Hear Res 34:119–126
Somlyo AV (1979) Bridging structures spanning the junctional gap at the triad of skeletal muscle. J Cell Biol 80:743–750
Ulfendahl M (1987) Motility in auditory sensory cells. Acta Physiol Scand 130:521–527
Zenner HP (1988) Motility of outer hair cells as an active, actin-mediated process. Acta Otolaryngol 105:39–44
Zenner HP, Zimmermann U, Schmitt U (1985) Reversible contraction of isolated mammalian cochlear hair cells. Hear Res 18:127–133
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arima, T., Kuraoka, A., Toriya, R. et al. Quick-freeze, deep-etch visualization of the ‘cytoskeletal spring’ of cochlear outer hair cells. Cell Tissue Res 263, 91–97 (1991). https://doi.org/10.1007/BF00318403
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318403