Summary
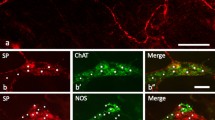
Immunoreactivity for calretinin, a calcium-binding protein, was studied in neurones in the guinea-pig small intestine. 26±1% of myenteric neurones and 12±3% of submucous neurones were immunoreactive for calretinin. All calretinin-immunoreactive neurones were also immunoreactive for choline acetyltransferase and hence are likely to be cholinergic. In the myenteric plexus, two subtypes of Dogiel type-I calretinin-immunoreactive neurones could be distinguished from their projections and neurochemical coding. Some calretinin-immunoreactive myenteric neurones had short projections to the tertiary plexus, and hence are likely to be cholinergic motor neurones to the longitudinal muscle. Some of these cells were also immunoreactive for substance P. The remaining myenteric neurones, immunoreactive for calretinin, enkephalin, neurofilament protein triplet and substance P, are likely to be orad-projecting, cholinergic interneurones. Calretinin immunoreactivity was also found in cholinergic neurones in the submucosa, which project to the submucosal vasculature and mucosal glands, and which are likely to mediate vasodilation. Thus, calretinin immunoreactivity in the guinea-pig small intestine is confined to three functional classes of cholinergic neurones. It is possible, for the first time, to distinguish these classes of cells from other enteric neurones.
Similar content being viewed by others
References
Ambache N, Freeman MA (1968) Atropine resistant longitudinal muscle spasms due to excitation of non-cholinergic neurons in Auerbachs plexus. J Physiol (Lond) 199:705–727
Bartho L, Holzer P, Leander S, Lembeck F (1989) Evidence for an involvement of substance P, but not cholecystokinin-like peptides in hexamethonium-resistant intestinal peristalsis. Neuroscience 28:211–217
Bornstein JC, Costa M, Furness JB, Lees GM (1984) Electrophysiology and enkephalin immunoreactivity of identified myenteric plexus neurons of guinea-pig small intestine. J Physiol (Lond) 351:313–325
Brookes SJH, Costa M (1990) Identification of enteric motor neurones which innervate the circular muscle of the guinea pig small intestine. Neurosci Lett 118:227–230
Costa M, Furness JB (1976) The peristaltic reflex: an analysis of nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol 294:47–60
Costa M, Furness JB (1983) The origins, pathways and terminations of neurons with VIP-like immunoreactivity in the guinea-pig small intestine. Neuroscience 8:665–676
Costa M, Furness JB, Llewellyn Smith JJ, Davies B, Oliver J (1980) An immunohistochemical study of the projections of somatostatin-containing neurons in the guinea-pig small intestine. Neuroscience 5:841–852
Costa M, Furness JB, Llewellyn-Smith IJ, Cuello AC (1981) Projections of substance P containing neurons with the guinea-pig small intestine. Neuroscience 6:411–424
Costa M, Furness JB, Gibbins IL (1986) Chemical coding of enteric neurons. Prog Brain Res 68:217–240
Costa M, Vitadello M, Dahl D (1989) The chemical coding of neurofilament immunoreactive enteric neurons in the guinea pig small intestine. Neurosci Lett [Suppl 34]:S74
Cuello AC, Galfre G, Milstein C (1979) Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci USA 67:3532–3536
Cuello AC, Milstein C, Coutore R, Wright B, Priestly JV, Jarvis J (1984) Characterization and immunocytochemical application of monoclonal antibodies against enkephalins. J Histochem Cytochem 9:947–957
Dogiel AS (1899) Über den Bau der Ganglien in den Geflechten des Darmes und der Gallenblase des Menschen und der Säugetiere. Arch Anat Physiol 130–158
Eckenstein F, Thoenen H (1982) Production of specific antisera and monoclonal antibodies to choline acetyltransferase: characterization and use for identification of cholinergic neurons. EMBO J 1:363–368
Ekblad E, Winther C, Ekman C, Håkanson R, Sundler F (1987) Projections of peptide-containing neurons in rat small intestine. Neuroscience 20:169–188
Erde SM, Sherman D, Gershon MD (1985) Morphology and serotonergic innervation of physiologically identified cells of the guinea pig's myenteric plexus. J Neurosci 5:617–633
Franco R, Costa M, Furness JB (1979) Evidence for the release of endogenous substance P from intestinal nerves. Naunyn Schmiedebergs Arch Pharmacol 306:195–201
Furness JB, Costa M (1987) The enteric nervous system. Churchill-Livingstone, Edinburgh
Furness JB, Costa M, Eckenstein F (1983a) Neurones localized with antibodies against choline acetyltransferase in the enteric nervous system. Neurosci Lett 40:105–109
Furness JB, Costa M, Emson PC, Håkanson R, Moghimzadeh E, Sundler F, Taylor IL, Chance RE (1983b) Distribution, pathways and reactions to drug treatment of nerves with neuropeptide Y-and pancreatic polypeptide-like immunoreactivity in the guinea pig digestive tract. Cell Tissue Res 234:71–92
Furness JB, Costa M, Miller RJ (1983c) Distribution and projections of nerves with enkephalin-like immunoreactivity in the guinea-pig small intestine. Neuroscience 8:653–664
Furness JB, Costa M, Gibbins IL, Llewellyn-Smith IJ, Oliver JR (1985) Neurochemically similar myenteric and submucous neurons directly traced to the mucosa of the small intestine. Cell Tissue Res 241:155–163
Furness JB, Keast JR, Pompolo S, Bornstein JC, Costa M, Emson PC, Lawson DEM (1988a) Immunohistochemical evidence for the presence of calcium-binding proteins in enteric neurons. Cell Tissue Res 252:79–87
Furness JB, Llewellyn Smith IJ, Bornstein JC, Costa M (1988b) Chemical neuroanatomy and the analysis of neuronal circuitry in the enteric nervous system. In: Björklund A, Hökfelt T, Owman C (eds) Handbook of chemical neuroanatomy. Elsevier, Amsterdam, pp 161–218
Grube D (1980) Immunohistochemistry of gastrin (G-) cells. II. Non-specific binding of immunoglobulins to G-cells by ionic interactions. Histochemistry 66:149–167
Hirst GDS, Spence I (1973) Calcium action potentials in mammalian peripheral neurones. Nature 243:54–56
Hirst GDS, Holman ME, Spence I (1974) Two types of neurones in the myenteric plexus of duodenum in the guinea pig. J Physiol (Lond) 236:303–326
Holzer P (1989) Ascending enteric reflex: multiple neurotransmitter systems and interactions. Am J Physiol 256:G540-G545
Iyer V, Bornstein JC, Costa M, Furness JB, Takahashi Y, Iwanaga T (1988) Electrophysiology of guinea pig myenteric neurons correlated with immunoreactivity for calcium-binding proteins. J Auton Nerv Syst 22:141–150
Katayama Y, Lees GM, Pearson GT (1986) Electrophysiology and morphology of vasoactive intestinal peptide immunoreactive neurones of the guinea-pig ileum. J Physiol (Lond) 378:1–11
Keast JR, Furness JB, Costa M (1984) Origins of peptides and norepinephrine nerves in the mucosa of the guinea pig small intestine. Gastroenterology 86:637–644
Kosterlitz HW, Lees GM (1964) Pharmacological analysis of intrinsic intestinal reflexes. Pharmacol Rev 16:301–339
Llewellyn-Smith IH, Furness JB, Gibbins IL, Costa M (1988) Quantitative ultrastructural analysis of enkephalin, substance P, VIP immunoreactive nerve fibres in the circular muscle of the guinea pig small intestine. J Comp Neurol 272:139–148
Morris JL, Gibbins IL, Furness JB, Costa M, Murphy R (1985) Co-localisation of NPY, VIP and dynorphin in non-noradrenergic axons of the guinea-pig uterine artery. Neurosci Lett 62:31–37
Morris JL, Gibbins IL, Murphy R (1986) Neuropeptide Y-like immunoreactivity is absent from most perivascular noradrenergic axons in a marsupial, the brush-tailed possum. Neurosci Lett 71:264–270
Neild TO, Shen KZ, Surprenant A (1990) Vasodilation of arterioles by acetylcholine released from single neurones in the guinea pig submucosal plexus. J Physiol (Lond) 420:247–265
Nishi S, North RA (1973) Intracellular recording from the myenteric plexus of the guinea pig ileum. J Physiol (Lond) 231:471–479
North RA (1973) The calcium-dependent slow after-hyperpolarisation in myenteric plexus neurones with tetrodotoxin-resistant action potentials. Br J Pharmacol 49:709–711
Pochet R, Blachier F, Malaisse W, Parmentier M, Pasteels B, Pohl V, Resibois A, Rogers J, Roman A (1989) Calbindin D28 in mammalian brain, retina, and endocrine pancreas: immunohistochemial comparison with calretinin. Adv Exp Med Biol 255:435–443
Rogers JH (1987) Calretinin: a gene for a novel calcium-binding protein expressed principally in neurons. J Cell Biol 105:1343–1353
Rogers JH (1989a) Two calcium-binding proteins mark many chick sensory neurons. Neuroscience 31:697–709
Rogers JH (1989b) Immunoreactivity for calretinin and other calcium-binding proteins in cerebellum. Neuroscience 31:711–721
Smith TK, Furness JB (1988) Reflex changes in circular muscle activity elicited by stroking the mucosa: an electrophysiological analysis of the isolated guinea pig ileum. J Auton Nerv System 25:205–218
Staines WA, Meister B, Melander T, Nagy J, Hökfelt T (1988) Three colour immunofluorescence histochemistry allowing triple labelling within a single section. J Histochem Cytochem 36:145–151
Steele PA, Costa M (1990a) Immunohistochemical identification of cholinergic neurons in the guinea pig ileum. Proc Aust Neurosci Soc 1:130
Steele PA, Costa M (1990b) Opioid-like immunoreactive neurons in secretomotor pathways of the guinea-pig ileum. Neuroscience 38:771–786
Stefanini M, DeMartino C, Zamboni L (1967) Fixation of ejaculated spermatozoa for electron microscopy. Nature 216:173–174
Tokimasa T, Cherubini E, North RA (1983) Nicotinic depolarisation activates calcium dependent gk in myenteric neurons. Brain Res 263:57–62
Tonini M, Costa M (1990) A pharmacological analysis of the neuronal circuitry involved in distension-evoked enteric excitatory reflex. Neuroscience 38:787–795
Vitadello M, Triban C, Fabris M, Gorio A, Schiaffino S (1986) Heterogeneity of rat neurofilament polypeptides revealed by a monoclonal antibody. J Neurochem 46:665–670
Wessendorf MW, Elde RP (1985) Characterization of an immunofluorescence technique for the demonstration of coexisting neurotransmitters within nerve fibres and terminals. Histochem Cytochem 33:984–994
Winsky L, Nakata H, Martin BM, Jacobowitz DM (1989) Isolation, partial amino acid sequence, and immunohistochemical localization of a brain-specific calcium-binding protein. Proc Natl Acad Sci USA 86:10139–10143
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brookes, S.J.H., Steele, P.A. & Costa, M. Calretinin immunoreactivity in cholinergic motor neurones, interneurones and vasomotor neurones in the guinea-pig small intestine. Cell Tissue Res 263, 471–481 (1991). https://doi.org/10.1007/BF00327280
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00327280