Summary
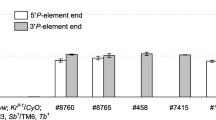
Choline acetyltransferase (ChAT, EC 2.3.1.6) catalyzes the production of the neurotransmitter acetylcholine, and is an essential factor for neurons to be cholinergic. We have analyzed regulation of the Drosophila ChAT gene during development by examining the β-galactosidase expression pattern in transformed lines carrying different lengths of 5′ flanking DNA fused to a lacZ reporter gene. The largest fragment tested, 7.4 kb, resulted in the most extensive expression pattern in embryonic and larval nervous system and likely reflects all the cis-regulatory elements necessary for ChAT expression. We also found that 5′ flanking DNA located between 3.3 kb and 1.2 kb is essential for the reporter gene expression in most of the segmentally arranged embryonic sensory neurons as well as other distinct cells in the CNS. The existence of negative regulatory elements was suggested by the observation that differentiating photoreceptor cells in eye imaginal discs showed the reporter gene expression in several 1.2 kb and 3.3 kb transformants but not in 7.4 kb transformants. Furthermore, we have fused the 5′ flanking DNA fragments to a wild type ChAT cDNA and used these constructs to transform Drosophila with a Cha mutant background. Surprisingly, even though different amounts of 5′ flanking DNA resulted in different spatial expression patterns, all of the positively expressing cDNA transformed lines were rescued from lethality. Our results suggest that developmental expression of the ChAT gene is regulated both positively and negatively by the combined action of several elements located in the 7.4 kb upstream region, and that the more distal 5′ flanking DNA is not necessary for embryonic survival and development to adult flies.
Similar content being viewed by others
References
Ashburner M (1989) Antibody staining of embryos. In “Drosophila — a laboratory manual”. Cold Spring Harbor Laboratory Press, Cold Spring, Harbor, New York, pp 214–216
Barber RP, Sugihara H, Lee M, Vaughn JE, Salvaterra PM (1989) Localization of Drosophila neurons that contain choline acetyltransferase messenger RNA: an in situ hybridization study. J Comp Neurol 280:533–543
Blochlinger K, Bodmer R, Jack JW, Jan LY, Jan YN (1988) Primary structure and expression of a product from cut, a locus involved in specifying sensory organ identity in Drosophila. Nature 333:629–635
Bodmer R, Barbel S, Shepherd S, Jack JW, Jan LY, Jan YN (1987) Transformation of sensory organs by mutations of the cut locus of Drosophila melanogaster. Cell 51:293–307
Bowtell DD, Lila T, Michael M, Hackett D, Rubin GM (1991) Analysis of the enhancer element that controls expression of sevenless in the developing Drosophila eye. Proc Natl Acad Sci USA 88:6853–6857
Buchner E, Buchner S, Crawford G, Mason WT, Salvaterra PM, Sattelle DB (1986) Choline acetyltransferase-like immunoreactivity in the brain of Drosophila melanogaster. Cell Tissue Res 246:57–62
Campos-Ortega JA (1988) Cellular interactions during early neurogenesis of Drosophila melanogaster. Trends Neurosci 11: 400–405
Campos-Ortega JA, Gateff EA (1976) The development of the ommatidial patterning in metamorphosed eye imaginal discs implants of Drosophila melanogaster. Roux's Arch Dev Biol 179:373–392
Campos-Ortega JA, Hartenstein V (1985) The embryonic development of Drosophila melanogaster. Springer, Berlin Heidelberg New York Tokyo
Carbini LA, Munoz-Maines VJ, Salvaterra PM (1990) Developmental expression of choline acetyltransferase mRNA in Drosophila. Neurochem Res 15:1089–1096
Carroll SD, Scott MP (1985) Localization of fushi tarazu protein during Drosophila embryogenesis. Cell 43:47–57
Chase BA, Kankel DR (1988) On the role of normal acetylcholine metabolism in the formation and maintenance of the Drosophila nervous system. Dev Biol 125:361–380
Dewhurst SA, McCaman RE, Kaplan WD (1970) The time course development of acetylcholine esterase and choline acetyltransferase in Drosophila melanogaster. Biochem Genet 4:499–508
Doe CQ, Smouse D, Goodman CS (1988a) Control of neuronal fate by the Drosophila segmentation gene even-skipped. Nature 333:376–378
Doe CQ, Hiromi Y, Gehring WJ, Goodman CS (1988b) Expression and function of the segmentation gene fushi tarazu during Drosophila neurogenesis. Science 239:170–175
Fonnum F (1975) A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem 24:407–409
Frasch M, Hoey T, Rushlow C, Doyle H, Levine M (1987) Characterization and localization of the even-skipped protein of Drosophila. EMBO J 6:749–759
Ghysen A, Dambly-Chaudiere C, Aceves E, Jan LY, Jan YN (1986) Sensory neurons and peripheral pathways in Drosophila embryos. Roux's Arch Dev Biol 195:281–289
Gorczyca MG, Hall JC (1984) Identification of a cholinergic synapse in the giant fiber pathway of Drosophila using conditional mutations of acetylcholine synthesis. J Neurogen 1:289–313
Gorczyca MG, Hall JC (1987) Immunohistochemical localization of choline acetyltransferase during development in the Cha ts mutants of Drosophila melanogaster. J Neurosci 7:1361–1369
Greenspan RJ (1980) Mutations of choline acetyltransferase and associated neural defects in Drosophila melanogaster. J Comp Physiol 137:83–92
Hall JC, Greenspan RJ, Kankel DR (1979) Soc Neurosci Symp 4:1–42
Hiromi Y, Kuroiwa A, Gehring WJ (1985) Control elements of the Drosophila segmentation gene fushi tarazu. Cell 43:603–613
Ibanez C, Persson H (1991) Localization of sequences determining cell type specificity and NGF responsiveness in the promoter region of the rat choline acetyltransferase gene. Eur J Neurosci 3:1309–1315
Ikeda K, Salvaterra PM (1989) Immunohistochemical study of a temperature sensitive choline acetyltransferase mutant of Drosophila melanogaster. J Comp Neurol 280:283–290
Itoh N, Slemmon JR, Hawke DH, Williamson R, Morita E, Itakura K, Roberts E, Shively JE, Crawford GD, Salvaterra PM (1986) Cloning of Drosophila choline acetyltransferase cDNA. Proc Natl Acad Sci USA 83:4081–4085
Jan YN, Ghysen A, Christoph I, Barbel S, Jan LY (1985) Formation of neuronal pathways in the imaginal discs of Drosophila melanogaster. J Neurosci 5:2453–2464
Johnson WA, McCormick CA, Bray SJ, Hirsh J (1989) A neuron-specific enhancer of the Drosophila dopa decarboxylase gene. Gene Dev 3:676–686
Kitamoto T, Ikeda K, Salvaterra PM (1992) Analysis of cis-regulatory elements in the 5′ flanking region of the Drosophila melanogaster choline acetyltransferase gene. J Neurosci 12:1628–1639
Lindsley D, Zimm G (1992) The genome of Drosophila melanogaster. Academic Press, San Diego, CA
Mac Donald PM, Ingham P, Struhl G (1986) Isolation, structure, and expression of even-skipped: a second pair-rule gene of Drosophila containing a homeobox. Cell 47:721–734
Rubin GM, Spradling AC (1982) Genetic transformation of Drosophila with transposable element vectors. Science 218: 348–353
Salvaterra PM, McCaman RE (1985) Choline acetyltransferase and acetylcholine levels in Drosophila melanogaster: a study using two temperature-sensitive mutants. J Neurosci 5:903–910
Sugihara H, Andrisani V, Salvaterra PM (1990) Drosophila choline acetyltransferase uses a non-AUG initiation codon and full length RNA is inefficiently translated. J Biol Chem 265:21714–21719
Sugihara H, Andrisani V, Salvaterra PM (1991) Genomic organization of Drosophila choline acetyltransferase gene. J Neurochem 57:1636–1642
Tajima Y, Salvaterra PM (1992) Positive and negative feedback regulation of choline acetyltransferase mRNA levels in Drosophila: a study using temperature-sensitive mutants and embryo cell culture. Mol Brain Res 13:213–221
Author information
Authors and Affiliations
Additional information
Correspondence to: P.M. Salvaterra
Rights and permissions
About this article
Cite this article
Kitamoto, T., Salvaterra, P.M. Developmental regulatory elements in the 5′ flanking DNA of the Drosophila choline acetyltransferase gene. Roux's Arch Dev Biol 202, 159–169 (1993). https://doi.org/10.1007/BF00365306
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00365306