Summary
-
1.
The development of vocalization and hearing was studied in Sri Lankan horseshoe bats (Rhinolophus rouxi) during the first postnatal month. The young bats were caught in a nursing colony of rhinolophids in which birth took place within a two week period.
-
2.
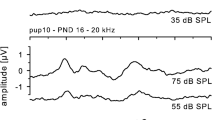
The new-born bats emitted isolation calls through the mouth. At the beginning these calls consisted of pure tones with frequencies below 10 kHz (Fig. 1). During the first postnatal week the call frequency increased to about 15 kHz, and the fundamental was augmented by two to four harmonics. No evoked potentials to pure tone stimuli could be elicited in the inferior colliculus of this age group, i.e., auditory processing at the midbrain level was not demonstrable.
-
3.
Evoked potentials were first recorded in the second week, broadly tuned to 15–45 kHz, with a maximum sensitivity between 15–25 kHz. In the course of the second week, however, higher frequencies up to 60 kHz became progressively incorporated into the audiogram (Fig. 3). The fundamental frequency of the multiharmonic isolation calls, emitted strictly through the mouth, increased to about 20 kHz.
-
4.
In the bats' third postnatal week an increased hearing sensitivity (auditory filter) emerged, sharply tuned at frequencies between 57 and 60 kHz (Fig. 4e). The same individuals were also the first to emit long constant frequency echolocation calls through the nostrils (Fig. 4c). The energy of the calls was arranged in harmonic frequency bands with the second harmonic exactly tuned to the auditory filter. These young bats continued to emit isolation calls through the mouth, which were, however, not harmonically related to the echolocation calls (Fig. 4b, d).
-
5.
During the fourth week, both the auditory filter and the matched echolocation pulses (the second harmonic) shifted towards higher frequencies (Fig. 5). During the fifth week the fundamental frequency of the calls was progressively attenuated, and both the second harmonic of the pulses and the auditory filter reached the frequency range typical for adult bats of 73–78 kHz (Fig. 6).
-
6.
The development of audition and vocalization is discussed with regard to possible interactions of both subsystems, and their incorporation into the active orientation system of echolocation.
Similar content being viewed by others
Abbreviations
- CF:
-
constant frequency
- FM:
-
frequency modulated
References
Aitkin LM, Moore DR (1975) Inferior colliculus II. Development of tuning characteristics and tonotopic organization in central nucleus of the neonatal cat. J Neurophysiol 38:1208–1216
Brown PE, Grinnell AD (1980) Echolocation ontogeny in bats. In: Busnel R-G, Fish JF (eds) Animal sonar systems. Plenum Press, New York, pp 355–377
Brown PE, Grinnell AD, Harrison JB (1978) The development of hearing in the Pallid bat,Antrozous pallidus. J Comp Physiol 126:169–182
Bruns V (1976) Peripheral auditory tuning for fine frequency analysis by the CF-FM bat,Rhinolophus ferrumequinum. I. Mechanical specializations of the cochlea. J Comp Physiol 106:77–86
Clopton BM, Winfield JA (1976) Effect of early exposure to patterned sound on unit activity in rat inferior colliculus. J Neurophysiol 39:1081–1089
Gallyas F (1979) Silver staining of myelin by means of physical development. Neurol Res 1:203–209
Gould E (1971) Studies of maternal-infant communication and development of vocalizations in the batsMyotis andEptesicus. Commun Behav Biol 5:263–313
Gould E (1975) Neonatal vocalization in bats of eight genera. J Mammal 56:15–29
Gould E (1979) Neonatal vocalizations of ten species of Malaysian bats (Megachiroptera and Microchiroptera). Am Zool 19:481–491
Grinnell AD, Hagiwara S (1972) Adaptations of auditory nervous system for echolocation (Studies in New Guinea bats). Z Vergl Physiol 76:41–81
Habersetzer J, Marimuthu G (1986) Ontogeny of sounds in the echolocating batHipposideros speoris. J Comp Physiol A 158:147–257
Harris DM, Dallos P (1984) Ontogenetic changes in frequency mapping of mammalian ear. Science 225:741–743
Konstantinov AI (1973) Development of echolocation in bats in postnatal ontogenesis. Period Biol 75:13–19
Kraus H-J, Aulbach-Kraus K (1981) Morphological changes in the cochlea of the mouse after the onset of hearing. Hearing Res 4:89–102
Matsumura S (1979) Mother infant communication in a Horseshoe bat (Rhinolophus ferrumequinum nippon): Development of vocalization. J Mammal 60:76–84
Mayr E (1974) Behavioural programs and evolutionary strategies. Am Sci 62:650–659
Moore DR (1983) Development of inferior colliculus and binaural audition. In: Romand R (ed) Development of auditory and vestibular system. Academic Press, New York London, pp 121–160
Neuweiler G (1970) Neurophysiologische Untersuchungen zum Echoortungssystem der Großen HufeisennaseRhinolophus ferrumequinum. Z Vergl Physiol 67:273–306
Neuweiler G (1977) Recognition mechanisms in echolocation of bats. In: Bullock TH (ed) Life Sci Res Rep 5:111–125
Neuweiler G, Metzner W, Heilmann U, Rübsamen R, Eckrich M, Costa HH (1987) Foraging behaviour in the rufous horseshoe bat,Rhinolophus rouxi, of Sri Lanka. Behav Ecol Sociobiol 20:53–67
Pujol R, Hilding D (1973) Anatomy and physiology of the onset of auditory function. Acta Otolaryngol 76:1–10
Relkin EM, Saunders JC (1980) Displacement of the malleus in neonatal gold hamster. Acta Otolaryngol 90:6–15
Rubel EW, Lippe WR, Ryals BM (1984) Development of the place principle. Ann Otol Rhinol Laryngol 93:609–615
Scheich H (1983) Sensorimotor interfacing. In: Ewert J-P, Capranica RR, Ingle DJ (eds) Advances in vertebrate neuroethology. Plenum Publishing Corporation, New York London, pp 7–14
Schnitzler H-U (1968) Die Ultraschall-Ortungslaute der Hufeisenfledermäuse (Chiroptera, Rhinolophidae) in verschiedenen Orientierungssituationen. Z Vergl Physiol 57:376–408
Schuller G, Pollak G (1979) Disproportionate frequency representation in the inferior colliculus of Doppler-compensating Greater Horseshoe bats: Evidence for an acoustic fovea. J Comp Physiol 132:47–54
Schuller G, Rübsamen R (1981) Laryngeal nerve activity during pulse emission in the CF-FM bat,Rhinolophus ferrumequinum. I. Superior laryngeal nerve (external motor branch). J Comp Physiol 143:317–321
Schuller G, Suga N (1976) Laryngeal mechanisms for the emission of CF-FM sounds in the Doppler-shift compensating bat,Rhinolophus ferrumequinum. J Comp Physiol 107:253–262
Schuller G, Beuter K, Schnitzler H-U (1974) Response to frequency shifted artificial echoes in the batRhinolophus ferrumequinum. J Comp Physiol 89:275–286
Sewell GD (1970) Ultrasonic communication in rodents. Nature 227:410
Shnerson A, Pujol R (1983) Development: Anatomy, electrophysiology and behaviour. In: Willott JF (ed) The auditory psychobiology of the house mouse. Thomas, Springfield, Illinois, pp 395–426
Shnerson A, Willot JF (1979) Development of inferior colliculus response properties in C57BL/6J mouse pubs. Exp Brain Res 37:373–386
Vater M (1987) Lightmicroscopic observations on cochlear development in horseshoe bats. Animal sonar systems. Helsingör Symposium, Plenum Press, New York London (in press)
Vater M, Feng AS, Betz M (1985) An HRP-study of the frequency-place map of the horseshoe bat cochlea: Morphological correlates of the sharp tuning to a narrow frequency band. J Comp Physiol A 157:671–686
Wiesel TN, Hubel DH (1963) Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol 26:1003–1017
Wiesel TN, Hubel DH (1965) Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol 28:1115–1156
Zippelius HM, Schleidt (1956) Ultraschall-Laute bei jungen Mäusen. Naturwissenschaften 43:502
Author information
Authors and Affiliations
Additional information
Supported by the Deutsche Forschungsgemeinschaft as part of Sonderforschungsbereich No. 114 and No. 204, München, and by the Joint Research Project of the University of Kelaniya and the University of München on the Biology and Ecology of Echolocating Bats in Sri Lanka
Rights and permissions
About this article
Cite this article
Rübsamen, R. Ontogenesis of the echolocation system in the rufous horseshoe bat,Rhinolophus rouxi (Audition and vocalization in early postnatal development). J. Comp. Physiol. 161, 899–913 (1987). https://doi.org/10.1007/BF00610231
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00610231