Summary
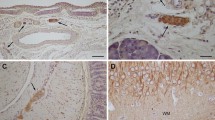
Schwann cells and endoneurial fibroblastlike cells were quantitatied for 30 weeks in both nonregenerating and freely regenerating, transected rat sciatic nerve. Immunocytochemical recognition of S-100 protein was used as a marker for Schwann cells and other immunocytochemical and histological methods in the differentiation of S-100 protein-negative endoneurial cells in cross sections of the distal stump 10 mm distal to the site of transection. A marked increase in the total number of cells was observed during the first 4 weeks after the injury in both operative groups. The quantitative relationships between cell populations remained essentially the same as in normal nerves, although the proliferation of the S-100 protein-negative cell population was proportionately slightly stronger when compared to the number of these cells in normal nerves. After the initial proliferation, a gradual decrease occurred in the total number of cells per cross section. This was most marked in the non-regenerating nerves, whereas in the regenerating nerves the decrease in cell number ceased at 16 weeks. The number of Schwann cells was 3.5 times as high as in the control nerves in this phase. The method used in the present study is less laborious than morphometry employing electron microscopy. Furthermore, electron microscopic characteristics of endoneurial cells are not always reliable after nerve trauma, because normal anatomical relationships have become disturbed. This study demonstrates that S-100 protein immunocytochemistry is useful in the study of traumatic lesions of peripheral nerve.
Similar content being viewed by others
References
Abercrombie M, Johnson ML (1946) Quantitative histology of Wallerian degeneration. I. Nuclear population in rabbit sciatic nerve. J Anat 80:37–50
Bradley WG, Asbury AK (1970) Duration of synthesis phase in neurilemma cells in mouse sciatic nerve during regeneration. Exp Neurol 26:275–282
Clark HB, Minesky JJ, Agrawal D, Agrawal HC (1985) Myelin basic protein and P2 protein are not immunohistochemical markers for Schwann cell neoplasms. Am J Pathol 121:96–101
Erlandson RA, Woodruff JM (1982) Peripheral nerve sheath tumors: an electron microscopic study of 43 cases. Cancer 49:273–287
Gibson JD (1979) The origin of the neural macrophage: a quantitative ultrastructural study of cell population changes during Wallerian degeneration. J Anat 129:1–19
Herrera GA, de Moraes HP (1984) Neurogenic sarcomas in patients with neurofibromatosis (von Recklinghausen's disease). Virchows Arch [A] 403:361–376
Isobe T, Ishioka N, Kocha T, Okuyama T (1983) Chemical structure and molecular evolution of S-100 proteins. In: Peeters H (ed) Proteins of the biological fluids, vol 30. Pergamon Press, New York pp 21–24
Isobe T, Okuyama T (1978) The amino acid sequence of S-100 protein (PAP I-b protein) and its relation to the calciumbinding proteins. Eur J Biochem 89:379–388
Jenq GB, Coggeshall RE (1985) Nerve regeneration through holey silicone tubes. Brain Res 361:233–241
Jurecka W, Ammerer HP, Lassmann H (1975) Regeneration of a transected peripheral nerve. An autoradiographic and electron microscopic study. Acta Neuropathol (Berl) 32:299–312
Kahn HJ, Marks A, Thom H, Baumal R (1983) Role of antibody to S-100 protein in diagnostic pathology. Am J Clin Pathol 79:341–347
Leder LD (1964) Über die selektive fermentcytochemische Darstellung von neutrophilen myeloischen Zellen und Gewebsmastzellen im Paraffinschnitt. Klin Wochenchr 42:553
Lugnegård H, Berthold C-H, Rydmark M (1984) Ultrastructural morphometric studies on regeneration of the lateral sural cutaneous nerve in the white rat after transection of the sciatic nerve. II. Regeneration after nerve suture and nerve grafting. Scand J Plast Reconstr Surg [Suppl] 20:27–64
Lugnegård H, Berthold C-H, Rydmark M (1984) Ultrastructural morphometric studies on regeneration of the lateral sural cutaneous nerve in the white rat after transection of the sciatic nerve. III. Regeneration after nerve suture in rats of different ages. Scand J Plast Reconstr Surg [Suppl] 20:65–85
Morris JH, Hudson AR, Weddell G (1972) A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. IV. Changes in fascicular microtopography, perineurium and endoneurial fibroblasts. Z Zellforsch 124:165–203
Nakajima T, Watanabe S, Sato Y, Kameya T, Hirota T, Shimosata Y (1982) An immunoperoxidase study of S-100 protein distribution in normal and neoplastic tissues. Am J Surg Pathol 6:715–727
Oldfors A (1980) Macrophages in peripheral nerves. An ultrastructural and enzyme histochemical study on rats. Acta Neuropathol (Berl) 49:43–49
Pellegrino RG, Spencer PS (1984) Schwann cell mitosis in response to regenerating peripheral axons in vivo. Brain Res 341:16–25
Peltonen J, Foidart J-M, Aho HJ (1984) Type IV and V collagens in von Recklinghausen's neurofibromas. An immunohistochemical and electrophoretical study. Virchows Arch [B] 47:291–301
Röyttä M, Salonen V, Peltonen J (1987) Reversible endoneurial changes after nerve injury. Acta Neuropathol (Berl) 73:323–329
Salonen V, Peltonen J, Röyttä M, Virtanen I (1987) Laminin in traumatized peripheral nerve. Basement membrane changes during degeneration and regeneration. J Neurocytol 16:713–720
Salzer JL, Bunge RP (1980) Studies of Schwann cell proliferation. I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J Cell Biol 84:739–752
Salzer JL, Bunge RP, Glaser L (1980) Studies of Schwann cell proliferation. III. Evidence for the surface localization of the neurite mitogen. J Cell Biol 84:767–778
Salzer JL, Williams AK, Glaser L, Bunge RP (1980) Studies of Schwann cell proliferation. II. Characterization of the stimulation and specificity of the response to a neurite membrane fraction. J Cell Biol 84:753–766
Scaravilli F (1984) Regeneration of the perineurium across a surgically induced gap in a nerve encased in plastic tube. J Anat 139:411–424
Schröder JM, Seiffert KE (1970) Die Feinstruktur der neuromatösen Neurotisation von nerventransplantaten. Virchows Arch [B] 5:219–235
Seiler N, Schröder JM (1970) Beziehungen zwischen Polyaminen und Nukleinsäuren. II. Biochemische und feinstrukturelle Untersuchungen am peripheren Nerven während des Wallerschen Degeneration. Brain Res 22:81–103
Sobue G, Brown MJ, Kim SU, Pleasure D (1984) Axolemma is a mitogen for human Schwann cells. Ann Neurol 15:449–452
Spicer SS (1965) Diamine methods for differentiating muscosubstances histochemically. J Histochem Cytochem 13:211–234
Sternberger LA (1979) The unlabelled antibody peroxidase-antiperoxidase (PAP) method. In: Immunocytochemistry. Wiley & Sons, New York, pp 297–321
Thomas GA (1948) Quantitative histology of Wallerian degeneration. II. Nuclear population in two nerves of different fibre spectrum. J Anat 82:135–145
Thomas PK (1966) The cellular response to nerve injury. 1. The cellular outgrowth from the distal stump of transected nerve. J Anat 100:287–303
Thomas PK, Jones DG (1967) The cellular response to nerve injury. 2. Regeneration of the perineurium after nerve section. J Anat 101:45–55
Thomas PK, Landon DN, King RHM (1984) Diseases of peripheral nerves. In: Adams JH, Corsellis JAN, Duchen LW (eds) Greenfield's neuropathology 4th edn. Arnold, London pp 838–843
Vizoso AD, Young JZ (1948) Internodal lenght and fibre diameter in developing and regenerating nerves. J Anat 82:110–134
Weidenheim KM, Campbell WG Jr (1986) Perineurial cell tumor. Immunohistochemical and ultrastructural characterization. Relationship to other peripheral nerve tumors with a review of the literature. Virchows Arch [A] 408:375–383
Weiss SW, Langloss JM, Enzinger FM (1983) Valde of S-100 protein in the diagnosis of soft tissue tumors with particular reference to benign and malignant Schwann cell tumors. Lab Invest 49:299–308
Author information
Authors and Affiliations
Additional information
Supported by a grant (to V. S.) from the Research and Science Foundation of Farmos and by institutional grants from the Sigrid Jusélius Foundation and the Turku University Foundation
Rights and permissions
About this article
Cite this article
Salonen, V., Aho, H., Röyttä, M. et al. Quantitation of Schwann cells and endoneurial fibroblast-like cells after experimental nerve trauma. Acta Neuropathol 75, 331–336 (1988). https://doi.org/10.1007/BF00687785
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00687785