Summary
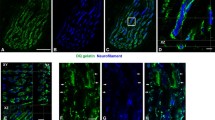
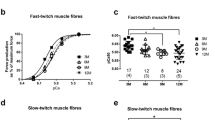
Little is known about the effects of exercise training on neuromuscular junction morphology in skeletal muscle. The objectives of this investigation were: 1) to determine if exercise training would elicit changes in neuromuscular junction morphology, 2) to determine if exercise training of different intensities would evoke specific changes in neuromuscular junction morphology, and 3) to determine whether changes in neuromuscular junction structure occur independently of changes in muscle fibre type and size. Twenty-four age and size matched male Sprague-Dawley rats were randomly assigned to three groups: high-intensity trained (HIT), low-intensity trained (LIT), or untrained. Neuromuscular junction morphology of the soleus muscle was determined via immunofluorescent staining. Presynaptic acetylcholine vesicles were visualized with SV-2 antibody in conjunction with fluorescein isothiocyanate labelled secondary antibody. Postsynaptic acetylcholine receptors were identified with rhodamine labelled α-bungarotoxin. Laser scanning microscopy was used to produce images of synapses, which were used to quantitate the following: total area of SV-2 and α-bungarotoxin staining, density of acetylcholine vesicles and receptors, structural complexity, and synaptic coupling. To visualize nerve terminal branching, a smaller number of neuromuscular junctions were stained with C-2 antibody, which reacts with a neurofilament epitope, in conjunction with fluorescein isothiocyanate labelled secondary antibody. Total length of branching, number of branches, average length of branches, and ratio of secondary to primary branches per neuromuscular junction were determined. Citrate synthase activity, fibre type composition and fibre cross-sectional areas of the soleus muscle were assessed to determine the presence of a training effect in that muscle. Results indicate that training did induce hypertrophy of the neuromuscular junction that was independent of muscle hypertorphy. Although the HIT and LIT groups exhibited similar hypertrophic responses of the neuromuscular junction, the HIT group displayed more dispersed synapses than the LIT group. Neither exercise training program, however, resulted in altered densities of acetylcholine vesicles or receptors, nor did training significantly change synaptic coupling. Nerve terminal branching was also affected by exercise training. Neuromuscular junctions from the HIT group demonstrated a greater total length of branching, average length per branch, and number of finer, or secondary, branches than those of the LIT group.
Similar content being viewed by others
References
Andonian, M. J. &Fahim, M. A. (1987) Effects of endurance exercise on the morphology of mouse neuromuscular junctions during ageing.Journal of Neurocytology 16, 589–99.
Armstrong, R. B. &Phelps, R. O. (1984) Muscle fiber type composition of the rat hind limb.The American Journal of Anatomy 171, 259–72.
Astrand, P. O., Hallback, I., Hedman, R. &Saltin, B. (1963) Blood lactate after prolonged severe exercise.Journal of Applied Physiology 18, 619–22.
Balice-Gordon, R. J. &Lichtman, J. W. (1990)In vivo visualization of the growth of pre- and postsynaptic elements of neuromuscular junctions in the mouse.Journal of Neuroscience 10, 894–908.
Booth, F. W. &Thomason, D. B. (1991) Molecular and cellular adaptation of muscle in response to exercise: perspective of various models.Physiological Reviews 71, 541–85.
Brooke, M. H. &Kaiser, K. K. (1970) Three myosin ATPase systems: the nature of the pH lability and sulfhydril dependence.Journal of Histochemistry and Cytochemistry 18, 670–2.
Brown, M. C., Hopkins, W. G., Keynes, R. J. &White, I. (1982) A comparison of early morphological changes at denervated and paralyzed endplates in fast and slow muscles of the mouse.Brain Research 248, 382–6.
Buckley, K. &Kelly, R. (1985) Identification of a transmembrane glycoprotein specific for secretory vesicles of neuronal and endocrine cells.Journal of Cell Biology 100, 1284–94.
Cardasis, C. A. (1983) Ultrastructural evidence of continued reorganization at the ageing (11–26 months) rat soleus neuromuscular junction.Anatomical Records 200, 399–415.
Cotman, C. W., Nietro-Sanpedro, M. &Harris, E. W. (1981) Synapse replacement in the nervous system of adult vertebrates.Physiological Reviews 61, 684–784.
Dahm, L. M. &Landmesser, L. T. (1988) The regulation of intramuscular nerve branching during normal development and following activity blockade.Developmental Biology 130, 621–44.
Dorlochtor, M., Irintchev, A., Brinkers, M. &Wernig, A. (1991) Effects of enhanced activity on synaptic transmission in mouse extensor digitorum longus muscle.Journal of Physiology 436, 283–92.
Fagg, G. E., Scheff, S. W. &Cotman, C. W. (1981) Axonal sprouting at the neuromuscular junction of adult and aged rats.Experimental Neurology 74, 847–54.
Gillespie, A. C., Fox, E. L. &Merola, A. J. (1982) Enzyme adaptations in rat skeletal muscle after two intensities of treadmill running.Medicine and Science in Sports and Exercise 14, 461–6.
Hill, R. R., Robbins, N. &Fang, Z. P. (1991) Plasticity of presynaptic and postsynaptic elements of neuromuscular junctions repeatedly observed in living adult mice.Journal of Neurocytology 20, 165–82.
Maltin, C. A., Delday, M. I., Baillie, A. G. S., Grubb, D. A. &Garlick, P. J. (1989) Fiber-type composition of nine rat muscles I. Changes during the first year of life.American Journal of Physiology 257, E823-E827.
Ogata, T. &Yamasaki, Y. (1985) The three-dimensional structure of motor endplates in different fiber types of rat intercostal muscle.Cell and Tissue Research 241, 465–72.
Rosenheimer, J. L. (1985) Effects of chronic stress and exercise on age-related changes in end-plate architecture.Journal of Neurophysiology 53, 1582–9.
Roy, R. R., Hirota, W. K., Kuehl, M. &Edgerton, V. R. (1985) Recruitment patterns in the rat hindlimb muscle during swimming.Brain Research 337, 175–8.
Smith, D. O. &Rosenheimer, J. L. (1982) Decreased sprouting and degeneration of nerve terminals of active muscles in aged rats.Journal of Neurophysiology 48, 100–9.
Srere, P. A. (1969) Citrate synthase.Methods in Enzymology 13, 3–5.
Stebbins, C. L., Schultz, E., Smith, R. T. &Smith, E. L. (1985) Effects of chronic exercise during aging on muscle and end-plate morphology in rats.Journal of Applied Physiology 58, 45–51.
Stephens, J. A. &Taylor, A. (1972) Fatigue of maintained voluntary muscle contraction in man.Journal of Physiology 220, 1–18.
Tomas, J., Ferre, J., Mayayo, E., Fenoll, R. &Brunet, R. (1987) Morphometric study of the neuromuscular synapses in the adult rat with special reference to the remodelling concept.Biology of the Cell 60, 133–44.
Van Der Meulen, J. H., Kuipers, H. &Drukker, J. (1991) Relationship between exercise-induced muscle damage and enzyme release in rats.Journal of Applied Physiology 71, 999–1004.
Waerhaug, O., Dahl, H. A. &Kardel, K. (1992) Different effects of physical training on the morphology of motor nerve terminals in the rat extensor digitorum longus and soleus muscles.Anatomy and Embryology 186, 125–8.
Wernig, A., Anzil, A. P. &Bieser, A. (1981) Formation and regression of synaptic contacts in adult muscle. InLesion-Induced Neuronal Plasticity in Sensorimotor Systems (edited byFlohr, H. &Precht, W.) Berlin: Springer.
Wernig, A., Pecot-Dechavassine, M. &Stover, H. (1980) Sprouting and regression of nerve at the frog neuromuscular junction in normal conditions and after prolonged paralysis with curare.Journal of Neurocytology 9, 227–303.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Deschenes, M.R., Maresh, C.M., Crivello, J.F. et al. The effects of exercise training of different intensities on neuromuscular junction morphology. J Neurocytol 22, 603–615 (1993). https://doi.org/10.1007/BF01181487
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01181487