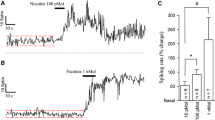
Summary
The significance of the cholinergic receptors within the noradrenergic nucleus locus coeruleus (LC) was analyzed by using single unit recording techniques and microiontophoretic drug application of various cholinergic and anticholinergic drugs. Physostigmine (25–50μg/kg), intravenously administered, caused increased firing of LC neurons and this effect was blocked by scopolamine but not by methylscopolamine. Also nicotine (10–50μg/kg i.v.) caused activation of LC neurons, an effect which was antagonized by the presumed nicotinic antagonist mecamylamine (8 mg/kg) as well as scopolamine (0.5 mg/kg i.v.). The cholinergic receptor within the LC showed specific muscarinic characteristics since microiontophoretic application of various muscarinic agonists caused excitation of LC units, whereas microiontophoretically applied nicotine had no effect. In addition, the acetylcholine (ACh)-induced excitation of the LC neurons was not blocked by nicotinic antagonists but was totally antagonized by the muscarinic agonist scopolamine. Scopolamine, when microiontophoretically applied onto the LC neurons, antagonized the stimulatory effect of systemically injected physostigmine but not that of nicotine. These results suggest that the stimulation of noradrenaline (NA) neurons in the LC by cholinergic drugs such as physostigmine is mediated via cholinergic, muscarinic receptors within this nucleus. The LC activation by nicotine is, however, an indirect effect, probably involving central ACh release and mediated via a non-cholinergic LC input.
Similar content being viewed by others
References
Amaral, D. G., Sinnamon, H. M. The locus coeruleus: Neurobiology of a central noradrenergic nucleus. Progress in Neurobiology9, 147–196 (1977).
Andén, N.-E., Bédard, P. Influences of cholinergic mechanisms on the function and turnover of brain dopamine. J. Pharm. Pharmac.23, 460–462 (1971).
Andén, N.-E., Wachtel, H. Increase in the turnover of brain dopamine by stimulation of muscarinic receptors outside the dopamine nerve treminals. J. Pharm. Pharmac.29, 435–437 (1977).
Armitage, A. K., Hall, G. H., Morrison, C. F. Pharmacological basis for the tobacco smoking habit. Nature217, 331–334 (1968).
Bartholini, G., Stadier, H. Cholinergic and GABA-ergic influence on the dopamine release in extrapyramidal centers. In: Chemical Tools in Catecholamine Research (Almgren, O., Carlsson, A., Engel, J., eds.), Vol. II, pp. 235–241. Amsterdam: North-Holland. 1975.
Bhatnagar, S. P. Studies related to the cholinergic influence on the accumulation and disappearance of monoamines in rat brain. Can. J. Physiol. Pharmacol.51, 893–899 (1973).
Bird, S., Aghajanian, G. K. Denervation supersensitivity in the cholinergic septo-hippocampal pathway: a microiontophoretic study. Brain Res.100, 355–370 (1975).
Bird, S., Kuhar, M. J. Iontophoretic application of opiates to the locus coeruleus. Brain Res.122, 523–533 (1977).
Butcher, L. L. Nature and mechanisms of cholinergic-monoaminergic interactions in the brain, Life Sci.21, 1207–1226 (1977).
Catchlove, R. F. H., Krnjević, K., Maretić, H. Similarity between effects of general anesthetics and dinitrophenol on cortical neurones. Can. J. Physiol. Pharmacol.50, 1111–1114 (1972).
Cedarbaum, J. M., Aghajanian, G. K. Activation of locus coeruleus neurons by peripheral stimuli: Modulation by a collateral inhibitory mechanism. Life Sci.23, 1383–1392 (1978).
Cheney, D. L., Lefevre, H. H., Racagni, G. Cholineacetyltransferase activity and mass fragmentographic measurement of ACh in specific nuclei and tracts of the rat brain. Neuropharmacology14, 801–809 (1975).
Corrodi, H., Fuxe, K., Hammer, W., Sjöqvist, F., Ungerstedt, U. Oxotremorine and central monoamine neurons. Life Sci.6, 2557–2566 (1967).
Corrodi, H., Fuxe, K., Lidbrink, P. Interaction between cholinergic and catecholaminergic neurones in rat brain. Brain Res.43, 397–416 (1972).
Davis, K. L., Hollister, L. E., Goodwin, F. K., Gordon, E. K. Neurotransmitter metabolites in the cerebrospinal fluid of man following physostigmine. Life Sci.21, 933–936 (1977).
Engberg, G., Svensson, T. H. Characterization of a cholinergic receptor on brain noradrenergic neurons: A microiontophoretic study. Neuroscience Letters, Suppl. 3, pp. 361 (1979).
Foot, S., Bloom, F. E.: Activity of locus coeruleus neurons in the unanesthetized squirrel monkey. Abstract in Catecholamines: Basic and Clinical Frontiers, 4th Int. Catecholamine Symposium, September 17 to 22, 1978, Pacific Grove, California, p. 49.
Fuxe, K., Hökfelt, T., Ungerstedt, U. Morphological and functional aspects of central monoamine neurons. Int. Rev. Neurobiol.13, 93–126 (1970).
Gebber, G. L. Neurogenic basis for the rise in blood pressure evoked by nicotine in the cat. J. Pharmacol. Exp. Ther.166, 255–263 (1969).
Guyenet, P. G., Aghajanian, G. K. Substance P and met-enkephalin in the locus coeruleus: Pharmacological evidence for independent sites of action. Eur. J. Pharmacol.53, 319–328 (1979).
Hall, G. H., Turner, D. M. Effects of nicotine on the release of3H-nor-adrenaline from the hypothalamus. Biochem. Pharmacol.21, 1829 to 1838 (1972).
Headley, P. M., Lodge, D., Biscoe, T. J. Acetylcholine receptors on Renshaw cells of the rat. Eur. J. Pharmacol.30, 252–359 (1975).
Kazic, T. Norepinephrine synthesis and turnover in brain: Acceleration by physostigmine. In: Frontiers in Catecholamine Research (Usdin, E., Snyder, S., eds.), pp. 897–899. New York: Pergamon Press. 1973.
Korf, J., Bunney, B. S., Aghajanian, G. K. Noradrenergic neurons: Morphine inhibition of spontaneous activity. Eur. J. Pharmacol.25, 165–169 (1974).
Krnjević, K. Chemical nature of synaptic transmission in vertebrates. Physiological Rev.54, 418–540 (1974).
Lindvall, O., Björklund, A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta physiol. scand., Suppl. 412, 1–48 (1974).
Maas, J. W. The effects of psychopharmacological agents on central nervous system amine metabolism in man. Ann. Rev. Pharmacol. Toxicol.17, 411–424 (1977).
Matsouka, I., Domino, E. F. Cholinergic modulation of single lateral geniculate neurons in the cat. Neuropharmacol.11, 241–251 (1972).
Morgan, W. W., Pfeil, K. A. Mecamylamine blockade of nicotine enhanced noradrenaline turnover in the rat brain. Life Sci.24, 417–420 (1979).
Morley, B. J., Lorden, J. F., Brown, G. B., Kemp, G. E., Bradley, R. J. Regional distribution of nicotinic acetylcholine receptor in rat brain. Brain Res.134, 161–166 (1977).
Mrsulja, B. B., Terzic, M., Varagic, V. M. The effect of physostigmine and neostigmine on the concentration of glucogen in various brain structures of the rat. J. Neurochem.15, 1329–1333 (1968).
Nygren, L., Olson, L. A new major projection from locus coeruleus: the main source of noradrenergic nerve terminals in the ventral and dorsal columns of the spinal cord. Brain Res.132, 85–93 (1977).
Przuntek, H., Phillppu, A. Reduced pressor response to stimulation of the locus coeruleus after lesion of the posterior hypothalamus. Naunyn-Schmiedeberg's Arch. Pharmacol.276, 119–122 (1973).
Redmond, D. E., Jr. Alterations in the function of the nucleus locus coeruleus: A possible model for studies of anxiety. In: Animal Models in Psychiatry and Neurology (Hanin, I., Usdin, E., eds.), pp. 293–305. Oxford-New York: Pergamon Press. 1977.
Salmoiraghi, G. C., Weight, F. Micromethods in neuropharmacology: An approach to the study of anesthetics. Anesthesiology28, 54–64 (1967).
Schmiterlöw, G. C., Hansson, E. The distribution of14C-nicotine. In: Tobacco Alkaloids and Related Compounds (von Euler, U. S., ed.), pp. 75–86. New York: Pergamon Press. 1965.
Svensson, T. H., Bunney, B. S., Aghajanian, G. K. Inhibition of both noradrenergic and serotonergic neurons in brain by theα-adrenergic agonist clonidine. Brain Res.92, 291–306 (1975).
Svensson, T. H., Engberg, G. Effect of nicotine on single cell activity in the noradrenergic nucleus locus coeruleus. Acta physiol. scand., Suppl. 479, 31–34 (1980).
Svensson, T. H., Thorén, P. Brain noradrenergic neurons in the locus coeruleus: Inhibition by blood volume load through vagal afferents. Brain Res.172, 174–178 (1979).
Svensson, T. H., Usdin, T. Feedback inhibition of brain noradrenaline neurons by tricyclic antidepressants:α-receptor mediation. Science202, 1089–1091 (1978).
Takigawa, M., Mogenson, G. J. A study of inputs to antidromically identified neurons of the locus coeruleus. Brain Res.135, 217–230 (1977).
Tasaki, K., Tsukuhara, U., Ito, S., Wayner, M. J., Yu, W. Y. A simple, direct and rapid method for filling microelectrodes. Physiol. Behav.3, 1009–1010 (1968).
Ungerstedt, U. Stereotoxic mapping of the monoamine pathways in the rat brain. Acta physiol. scand., Suppl. 367, 1–48 (1971).
Varagic, V. M. The action for eserine on the blood pressure of the rat. Br. J. Pharmacol. Chemother.10, 349–353 (1955).
Varagic, V. M., Krstic, M. Adrenergic activation by anticholinesterases. Pharmacol. Rev.18, 799–800 (1966).
Ward, D. G., Gunn, C. G. Locus coeruleus complex: elicitation of a presser response and a brain stem region necessary for its occurrence. Brain Res.107, 401–406 (1976).
Westfall, T. C. Effects of nicotine and other drugs on the release of3H-norepinephrine and3H-dopamine from rat brain slides. Neuropharmacol.13, 639–700 (1974).
Author information
Authors and Affiliations
Additional information
Parts of these results were reported at a symposium on “The Effects of Nicotine on Central Nervous System” in Stockholm, Sweden, November 28–29, 1978, and at the Third European Neuroscience Meeting, Rome, Italy, September 11–14, 1979.
Rights and permissions
About this article
Cite this article
Engberg, G., Svensson, T.H. Pharmacological analysis of a cholinergic receptor mediated regulation of brain norepinephrine neurons. J. Neural Transmission 49, 137–150 (1980). https://doi.org/10.1007/BF01245220
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01245220