Summary
-
1.
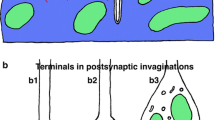
Photoreceptor terminals in the fliesMusca domestica andDrosophila melanogaster have been reconstructed in three dimensions from serial EM to reveal the surface distributions of afferent tetrad synapses.
-
2.
The terminals are cylindrical and surround two target cells; they have synaptic sites distributed along their length and around their circumference, except for a strip along the face that lies furthest away from the target cells.
-
3.
Over their inner faces, the terminals have presynaptic sites that are distributed evenly.
-
4.
The distribution of sites in maps plotted from reconstructed membrane surfaces was examined by quadrat analyses. The frequency of sites per quadrat division was not Poissonian, i.e. was non-random. Thus, some form of site selection must exist during synaptogenesis.
-
5.
The sites were shown by variance ratio analysis to be regular (evenly dispersed, not clustered). This suggests that some form of interaction exists, so as to reduce the probability that a synapse will form close to an already existing synaptic site.
-
6.
Distances between nearest-neighbour pairs of synapses had a closest minimum spacing of about 0.8 μm inMusca that was violated by about 5% of pairs, whereas the corresponding distances were about 0.2 μm shorter inDrosophila, which had 13% of pairs situated closer together than 0.8 μm.
-
7.
During synaptogenesis, either initially in the pupa or later in the adult, the probability that a synapse will form is therefore effectively zero within these distances from an existing synaptic site, perhaps through an inhibitory influence exerted by the latter. The nearest-neighbour distances are normally distributed.
-
8.
Unlike the distribution of presynaptic sites, the distribution of postsynaptic sites over the surfaces of the dendrites of the target cells is not even. Although not studied in detail, the corresponding nearest-neighbour distances are much smaller, as little as 0.1 μm. Thus the wider spacing seen between sites over the receptor terminals is a function of the presynaptic cells, and not of their postsynaptic partners, and implies the existence of interactions between synaptic sites.
Similar content being viewed by others
References
Bennett, M. R., and Pettigrew, A. G. (1974). The formation of synapses in straited muscle during development.J. Physiol. 241:515–545.
Bennett, M. R., and Pettigrew, A. G. (1976). The formation of neuromuscular synapses.Cold Spring Harbor Symp. Quant. Biol. 40:409–424.
Brandstätter, J. H., Shaw, S. R., and Meinertzhagen, I. A. (1991a). Terminal degeneration and synaptic disassembly following receptor photoablation in the retina of the fly's compound eye.J. Neurosci. 11:1930–1941.
Brandstätter, J. H., Shaw, S. R., and Meinertzhagen, I. A. (1991b). Invagination of presynaptic ribbons in the fly's optic lobe following loss of their target neuron.Proc. Roy. Soc. Lond. B 245:13–22.
Boycott, B. B., and Hopkins, J. M. (1991). Cone bipolar cells and cone synapses in the primate retina.Vis. Neurosci. 7:49–60.
Burry, R. W., Kniss, D. A., and Scribner, L. R. (1984). Mechanisms of synapse formation and maturation.Curr. Topics Res. Synapses 1:1–51.
Canal, I., Fariñas, I., Gho, M., and Ferrús, A. (1994). The presynaptic cell determines the number of synapses in theDrosophila optic ganglia.Eur. J. Neurosci. 6:1423–1431.
Cotman, C. W., and Nieto-Sampedro, M. (1984). Cell biology of synaptic plasticity.Science 225:1287–1294.
Cotman, C. W., Nieto-Sampedro, M., and Harris, E. W. (1981). Synapse replacement in the nervous system of adult vertebrates.Physiol. Rev. 61:684–784.
Dowling, J. E., and Boycott, B. B. (1966). Organization of the primate retina: electron microscopy.Proc. Roy. Soc. Lond. B 166:80–111.
Fischbach, K.-F., and Dittrich, A. P. M. (1989) The optic lobe ofDrosophila melanogaster. I. A Golgi analysis of wild-type structure.Cell Tissue Res. 258:441–475.
Florey, E., and Cahill, M. A. (1982). The innervation pattern of crustacean skeletal muscle. Morphometry and ultrastructure of terminals and synapses.Cell Tissue Res. 224:527–541.
Fröhlich, A. (1986). Freeze-fracture study of an invertebrate multiple-contact synapse: The fly photoreceptor tetrad.J. Comp. Neurol. 241:311–326.
Fröhlich, A. (1996). The making of a neuropile: Preferential adjacency among neurons and its consequences for their synapses. (in preparation).
Fröhlich, A., and Meinertzhagen, I. A. (1982). Synaptogenesis in the first optic neuropile of the fly's visual system.J. Neurocytol. 11:159–180.
Fröhlich, A., and Meinertzhagen, I. A. (1983). Quantitative features of synapse formation in the fly's visual system. I. The presynaptic photoreceptor terminal.J. Neurosci. 3:2336–2349.
Fröhlich, A., and Meinertzhagen, I. A. (1987). Regulation of synaptic frequency: Comparison of the effects of hypoinnervation with those of hyperinnervation in the fly's compound eye.J. Neurobiol. 18:343–357.
Gottlieb, D. I., and Cowan, W. M. (1973). Autoradiographic studies of the commissural and ipsilateral associational connections of the hippocampal and dentate gyrus of the rat. I. The commissural connections.J. Comp. Neurol. 149:393–422.
Govind, C. K., and Chiang, R. G. (1979). Correlation between presynaptic dense bodies and transmitter output at lobster neuromuscular terminals by serial section electron microscopy.Brain Res. 161:377–388.
Hall, Z. W., and Sanes, J. R. (1993). Synaptic structure and development: The neuromuscular junction.Cell 72/Neuron 10:99–121.
Hardie, R. C. (1987). Is histamine a neurotransmitter in insect photoreceptors?J. Comp. Physiol. A 161:201–213.
Hauser-Holschuh, H. (1975).Vergleichend quantitative Untersuchungen an den Sehganglien der Fliegen Musca domesticaUnd Drosophila melanogaster, Dissertation, Eberhard-Karls-Universität, Tübingen.
Hu, X. (1993).Three-Dimensional Reconstruction of Optic Lobe Interneurons and Their Synapses in the Flies Muscaand Drosophila, M.Sc. thesis, Department of Biology, Dalhousie University, Halifax.
Kelly, R. B. (1993). Storage and release of neurotransmitters.Cell 72/Neuron 10 (Suppl.):43–53.
Kershaw, K. A., and Looney, J. H. (1985).Quantitative and Dynamic Plant Ecology, Edward Arnold, London.
Koenig, J. H., and Ikeda, K. (1989). Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval.J. Neurosci. 9:3844–3860.
Kuffler, D., Thompson, W., and Jansen, J. K. S. (1977). The elimination of synapses in multiply-innervated skeletal muscle fibres of the rat: Dependence on distance between end-plates.Brain Res. 138:353–358.
Laughlin, S. B. (1973). Neural integration in the first optic neuropile of dragonflies I. Signal amplification in dark-adapted second-order neurons.J. Comp. Physiol. 84:335–355.
Laughlin, S. B., Howard, J., and Blakeslee, B. (1987). Synaptic limitations to contrast coding in the retina of the blowflyCalliphora.Proc. Roy. Soc. Lond. B 231:437–467.
Lavidis, N. A., and Bennett, M. R. (1992). Probabilistic secretion of quanta from visualized sympathetic nerve varicosities in mouse vas deferens.J. Physiol. 454:9–26.
Lømo, T., and Jansen, J. K. S. (1980). Requirements for the formation and maintenance of neuromuscular connections. InNeural Development, Part II (R. K. Hunt, Ed.), Academic Press, New York, pp. 253–281.
Mariani, J., and Changeux, J.-P. (1981). Ontogenesis of olivocerebellar relationships I. Studies by intracellular recordings of the multiple innervation of Purkinje cells by climbing fibers in the developing rat cerebellum.J. Neursoci. 1:696–702.
Meinertzhagen, I. A. (1984). The rules of synaptic assembly in the developing insect lamina. InPhotoreception and Vision in Invertebrates (M. A. Ali, Ed.), NATO Advanced Science Institute, Vol. 74, Plenum Press, New York, pp. 635–660.
Meinertzhagen, I. A. (1989). Fly photoreceptor synapses: Their development, evolution, and plasticity.J. Neurobiol. 20:276–294.
Meinertzhagen, I. A. (1993). The synaptic populations of the fly's optic neuropil and their dymamic regulation: Parallels with the vertebrate retina.Prog. Retinal Res. 12:13–39.
Meinertzhagen, I. A. (1994). The early causal influence of cell size upon synaptic number: The mutantgigas ofDrosophila.J. Neurogenet. 9:157–176.
Meinertzhagen, I. A., and Fröhlich, A. (1983). The regulation of synapse formation in the fly's visual system.Trends Neurosci. 6:223–228.
Meinertzhagen, I. A., and Hanson, T. E. (1993). The development of the optic lobe. InThe Development of Drosophila melanogaster (M. Bate and A. Martinez Arias, Eds.), Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 1363–1491.
Meinertzhagen, I. A., and O'Neil, S. D. (1991). Synaptic organization of columnar elements in the lamina of the wild type inDrosophila melanogasten.J. Comp. Neurol. 305:232–263.
Nicol, D., and Meinertzhagen, I. A. (1982). An analysis of the number and composition of the synaptic populations formed by photoreceptors of the fly.J. Comp. Neurol. 207:29–44.
Palay, S. L. and Chan-Palay, V. (1974).Cerebellar Cortex: Cytology and Organization, Springer-Verlag, New York.
Raisman, G. (1985). Synapse formation in the septal nuclei of adult rats. InSynaptic Plasticity (C. W. Cotman, Ed.), Guilford Press, New York, pp. 13–38.
Rao, R., Buchsbaum, G., and Sterling, P. (1994). Rate of quantal transmitter release at the mammalian rod synapse.Biophys. J. 67:57–63.
Ramaswami, M., Krishman, K. S., and Kelly, R. B. (1994). Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions.Neuron 13:363–375.
Raviola, E., and Gilula, N. B. (1975). Intramembrane organization of specialized contacts in the outer plexiform layer of the retina: A freeze-fracture study in monkeys and rabbits.J. Cell Biol. 65:192–222.
Ribchester, R. R., and Barry, J. A. (1994). Spatialversus consumptive competition at polyneuronally innervated neuromuscular junctions.Exp. Physiol. 79:465–494.
Ribi, W. A. (1976). A Golgi-electron microscope method for insect nervous tissue.Stain Technol. 51:13–16.
Ryan, T. A., Joiner, B. L., and Ryan, B. F. (1976).Minitab Student Handbook, Duxbury Press, North Scituate, MA.
Rybak, J., and Meinertzhagen, I. A. (1996). The effects of light reversals on photoreceptor synaptogenesis.Europ. J. Neurosci. (submitted).
Saint Marie, R. L., and Carlson, S. D. (1982). Synaptic vesicle activity in stimulated and unstimulated photoreceptor axons in the housefly. A freeze-fracture study.J. Neurocytol. 11:747–761.
Sargent, P. B. (1983). The number of synaptic boutons terminating onXenopus cardiac ganglion cells is directly correlated with cell size.J. Physiol. 343:85–104.
Sterling, P. (1990). Retina. InThe Synaptic Organization of the Brain, 3rd ed., Oxford University Press, New York, pp. 170–213.
Strausfeld, N. J., and Nässel, D. R. (1981). Neuroarchitectures serving compound eyes of Crustacea and insects. InHandbook of Senory Physiology, Vol. VII/6B. Comparative Physiology and Evolution of Vision in Invertebrates (H. Autrum, Ed.), Springer-Verlag, Berlin, pp. 1–132.
Streichert, L. C., and Sargent, P. B. (1989). Bouton ultrastructure and synaptic growth in a frog autonomic ganglion.J. Comp. Neurol. 281:159–168.
Trimble, W. S., Linial, M., and Scheller, R. H. (1991). Cellular and molecular biology of the presynaptic nerve terminal.Annu. Rev. Neurosci. 14:93–122.
White, J. G. (1985). Neuronal connectivity inCaenorhabditis elegans.Trends Neurosci. 8:277–283.
Winslow, J. L., Cooper, R. L., Govind, C. K., Pearce, J., Marin, L., and Atwood, H. L. (1994). Close presynaptic active zones may enhance facilitation.Soc. Neurosci. Abstr. 20:1339.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meinertzhagen, I.A., Hu, X. Evidence for site selection during synaptogenesis: The surface distribution of synaptic sites in photoreceptor terminals of the fliesMusca andDrosophila . Cell Mol Neurobiol 16, 677–698 (1996). https://doi.org/10.1007/BF02151904
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02151904