Summary
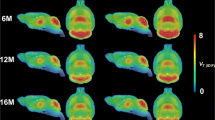
D1-dopamine receptor binding in the brain was determined by positron emission tomography (PET) in five patients with Huntington's disease, in one asymptomatic gene carrier and in five control subjects. [11C] SCH 23390 was used as the radioligand. Brain morphology was recorded by MRI. The patients who all had a mild to moderate functional impairment showed an almost 50% reduction of putamen volume as well as D1-dopamine receptor density as compared to the controls. The total D1-dopamine receptor number in the putamen was reduced by 75% in the patient group. A similar reduction was found for the caudate nucleus. The asymptomatic gene carrier had volume and density values in the lower range of the control subjects. In the frontal neocortex there also tended to be a reduced D1-dopamine receptor binding in the symptomatic patients. The results indicate that [11C] SCH 23390 binding in combination with MRI can be used as a sensitive marker for early brain degeneration in Huntington's disease. This marker may be useful to monitor the pathophysiological effect of the disease gene and also to follow therapeutic interventions aiming at preventing the degenerative process.
Similar content being viewed by others
References
Berent S, Giordani B, Lehtinen S, Markel D, Penney J, Buchtel H, Starosta-Rubinstein S, Hichwa R B AYA (1988) Positron emission tomographic scan investigations of Huntington's disease: Cerebral metabolic correlates of cognitive function. Ann Neurol 23:541–546
Bergström M, Boëthius J, Eriksson L, Greitz T, Ribbe T, Widén L (1981) Head fixation device for reproducible position alignment in transmission CT and positron emission tomography. J Comput Assist Tomogr 5:136–141
Brandt J, Folstein S, Wong D, Links J, Dannals R, McDonnell-Sill, Starkstein S, Anders P, Strauss M, Tune L, Wagner HJ, Folstein M (1990) D2 receptors in Huntington's disease: Positron emission tomography findings and clinical correlates. J Neuropsychiatry Clin Neurosci 2:20–27
Cortés R, Camps M, Gueye B, Probst A, Palacios JM (1989) Dopamine receptors in human brain: autoradiographic distribution of D1 and D2 sites in Parkinson syndrome of different etiology. Brain Res 483:30–38
Cross A, Rosser M (1983) Dopamine D-1 and D-2 receptors in Huntingtons desease. Eur J Pharmacol 88:223–229
Farde L, Hall H, Ehrin E, Sedvall G (1986) Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science 231:258–261
Farde L, Halldin C, Stone-Elander S, Sedvall G (1987) PET analysis of human dopamine receptor subtypes using11C-SCH 23390 and11C-raclopride. Psychopharmacology 92:278–284
Farde L, Pauli S, Hall H, Eriksson L, Halldin C, Högberg T, Nilsson L, Sjögren I, Stone-Elander S (1988) Stereoselective binding of11C-raclopride binding in living human brain — a search for extrastriatal D2-dopamine receptors by PET. Psychopharmacology 94:471–478
Ferrante R, Kowall N, Beal M, Martin J, Bird E, Richardson EJ (1987) Morphologic and histochemical characteristics of a spared subset of striatal neurons in Huntington's disease. J Neuropathol Exp Neurol 46:12–27
Ferrante R, Kowall N, Richardson EJ (1991) Proliferative and degenerative changes in striatal spiny neurons in Huntington's disease: a combined study using the section-Golgi method and calbindin D28k immunocytochemistry. Neuroscience 11:3877–3887
Filloux F, Wagster M, Folstein S, Price D, Hedreen J, Dawson T, Wamsley J (1990) Nigral dopamine type-1 receptors are reduced in Huntington's disease: A postmortem autoradiographic study using [3H] SCH 23390 and correlation with [3H] forskolin binding. Exp Neurol 110:219–227
Folstein M, Folstein S, McHugh P (1975) “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinican. J Psychiatr Res 2:189–198
Folstein S, Jensen B, Leigh R, Folstein M (1983) The measurement of abnormal movement: Methods developed for Huntington's disease. Neurobehav Toxicol Teratol 5:605–609
Gerfen C, Baimbridge K, Miller J (1985) The neostriatal mosaic compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc Natl Acad Sci USA 82:8780–8784
Goto S, Hirano A, Rojas-Corona R (1989) An immunohistological investigation of the human neostriatum in Huntington's disease. Ann Neurol 25:298–304
Graveland (1985a) Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntingtons desease. Science 227:770–773
Graveland (1985b) Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntingtons desease. Science 227:770–773
Hall H, Farde L, Sedvall G (1988) Human dopamine receptor subtypes — in vitro binding analysis using3H-SCH 23390 and3H-raclopride. J Neural Transm 73:7–21
Halldin C, Stone-Elander S, Farde L, Ehrin E, Fasth K-J, Långström B, Sedvall G (1986) Preparation of11C-labelled SCH 23390 for the in vivo study of dopamine D-1 receptors using positron emission tomography. Appl Radiat Isot 37:1039–1043
Harris G, Pearlson G, Peyser C, Aylward E, Roberts J, Barta P, Chase G, Folstein S (1992) Putamen volume reduction on magnetic resonance imaging exceeds caudate changes in mild Huntington's disease. Ann Neurol 31:69–75
Hasselbach S, Oberg G, Sorensen S, Andersen A, Waldemar G, Schmidt J, Fenger K, Paulson O (1992) Reduced regional cerebral blood flow in Huntington's Disease studied by SPECT. J Neurol Neurosurg Psychiatry 55:11
Hayden H, Hewitt J, Stoessl A, Clark C, Ammann W, Martin W (1987) The combined use of positron emission tomography and DNA polymorphisms for preclinical detection of Huntington's disease. Neurology 37:1441–1447
Hägglund J, Aquilonius S-M, Eckernäs S-Å, Hartvig P, Lundquist H, Gullberg P, Långström B (1987) Dopamine receptor properties in Parkinson's disease and Huntington's chorea evaluated by positron emission tomography using11C−N-methylspiperone. Acta Neurol Scand 75:87–94
Joyce J, Lexow N, Bird E, Winokur A (1988) Organization of dopamine D1 and D2 receptors in human striatum: Receptor autoradiographic studies in Huntington's disease and schizophrenia. Synapse 2:546–557
Kowall N, Ferrante R, Beal M, Richardsson EJ, Sofreniew M, Cuello A, Martin J (1987) Neuropeptide Y,-somatostatin and NADPH diaphorase in human striatum: a combined immunocytochemical and enzyme histochemical study. Neuroscience 20:817
Leenders K, Frackowiak R, Quinn N (1986) Brain energy metabolism and dopaminergic functions in Huntington's disease measured in vivo using positron emission tomography. Mov Disord 1:69–77
Litton JE, Holte S, Eriksson L (1990) Evaluation of the Karolinska new positron camera system; the Scanditronix PC2048-15B. IEEE Trans Nucl Sci 37:743–748
Martin J, Gusella J (1986) Huntington's disease: pathogenesis and management. N Engl J Med 315:1267
Mazziotta J, Phelps M, Pahl J, Huang S-C, Baxter L, Riege W, Hoffman J, Kuhl D, Lanto A, Wapenski J, Markham C (1987) Reduced cerebral glucose metabolism in asymptomatic subjects at risk for Huntington's disease. New Engl J Med 316: 357–362
Meador-Woodruff JH, Mansour A, Healy DJ, Kuehn R, Zhou Q-Y, Bunzow JR, Akil H, Civelli O, Watson Jr. SJ (1991) Comparison of the distributions of D1-dopamine receptor mRNAs in rat brain. Neuropsychopharmacology 5:231–242
Quimet C, Miller P, Hemmings HJ, Walaas S, Greengard P (1984) DARPP-32, a dopamine and adenosine 3′∶5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. Neuroscience 4:114–124
Reisine T, Fields J, Stern L (1977) Alterations in dopaminergic receptors in Huntington's disease. Life Sci 21:1123–1128
Richfield E, O'Brien C, Eskin T, Shoulson I (1991) Heterogeneous dopamine receptor changes in early and late Huntington's disease. Neurosci Lett 132:121–126
Sedvall G, Farde L, Stone-Elander S, Halldin C (1986) Dopamine D1-receptor binding in the living human brain. In: G.R. Breese and I. Creese (eds) Neurobiology of central D1-dopamine receptors. Plenum, New York, pp 119–124
Seeman P, Bzowej N, Guan H, Beregeron C, Reynolds G, Bird E, Riederer P, Jellinger K, Tourteotte W (1987) Human brain D1- and D2-dopamine receptors in schizophrenia, Alzheimer's, Parkinson's, and Huntington's diseases. Neuropsychopharmacology 1:5–15
Suhara T, Fukuda H, Inoue O, Itoh T, Suzuki K, Yamasaki T, Tateno Y (1991) Age-related changes in human D1-dopamine receptors measured by positron emission tomography. Psychopharmacology 103:41–45
Swahn C-G, Farde L, Halldin C, Sedvall G (1992) Ligand metabolites in plasma during PET studies with the11C-labelled dopamine antagonists, raclopride, SCH 23390 and N-methylspiroperidol. Hum Psychopharmacol 7:97–103
Swahn C-G, Farde L, Halldin C, Sedvall G (1993) Metabolism of the PET ligand [11C] SCH 23390. Identification of two metabolites with HPLC. Hum Psychopharmacol (in press)
The Huntington's Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntinton's disease chromosomes. Cell 72: 971–983
Vonsattel J-P, Meyers R, Stevens T, Ferrante R, Bird E, Richardsson EJ (1985) Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol 44:559–577
Walaas S, Asswar D, Greengard P (1983) A dopamine and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature 301:69–71
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sedvall, G., Karlsson, P., Lundin, A. et al. Dopamine D1 receptor number — a sensitive PET marker for early brain degeneration in Huntington's disease. Eur Arch Psychiatry Clin Nuerosci 243, 249–255 (1994). https://doi.org/10.1007/BF02191583
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02191583