Summary
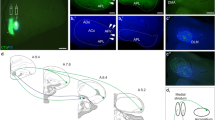
The possibility that certain of the afferents of the primate amygdaloid complex use an excitatory amino acid transmitter was evaluated by injecting D-[3H]-aspartate into the amygdala of twoMacaca fascicularis monkeys. The distribution of D-[3H]-aspartate labeled neurons was compared with those labeled with the nonselective retrograde tracer WGA-HRP injected at the same location as the isotope. Retrogradely labeled cells of both types were observed in a variety of cortical and subcortical structures and in discrete regions within the amygdala. D-[3H]-aspartate labeled neurons were observed in layers III and V of the frontal, cingulate, insular and temporal cortices. In the hippocampal formation, heavily labeled cells were observed in the CA1 region and in the deep layers of the entorhinal cortex. Of the subcortical afferents, the claustrum and the midbrain peripeduncular nucleus contained the greatest number of D-[3H]-aspartate labeled cells. Subcortical afferents that are not thought to use excitatory amino acids, such as the cholinergic neurons of the basal nucleus of Meynert, did not retrogradely transport the isotope. Within the amygdala, the most conspicuous labeling was in the paralaminar nucleus which forms the rostral and ventral limits of the amygdala. When the D-[3H]-aspartate injection involved the basal nucleus, many labeled cells were also observed in the lateral nucleus. Retrograde transport of D-[3H]-aspartate injected into the amygdala, therefore, appears to demonstrate a subpopulation of inputs that may use an excitatory amino acid transmitter.
Similar content being viewed by others
Abbreviations
- 36pl Area:
-
36 (lateral portion of polar region)
- 36pm Area:
-
36 (medial portion of polar region)
- 36r Area:
-
36 (rostral portion)
- A1:
-
Primary auditory field
- ABmg:
-
Accessory basal nucleus of the amygdala (magnocellular portion)
- ABpc:
-
Accessory basal nucleus of the amygdala (parvicellular portion)
- ac:
-
Anterior commissure
- amts:
-
Anterior middle temporal sulcus
- as:
-
Arcuate sulcus
- B:
-
Basal nucleus of the amygdala
- BNM:
-
Basal nucleus of Meynert
- CA1:
-
Field CA1 of the Hippocampus
- cc:
-
Corpus callosum
- C:
-
Central nucleus of the amygdala
- CD:
-
Caudate nucleus
- Cl:
-
Claustrum
- cs:
-
Cingulate sulcus
- EC:
-
Entorhinal cortex
- G:
-
Gustatory cortex
- H:
-
Hippocampus
- I:
-
Intercalated nucleus of the amygdala
- Ia:
-
Agranular insular cortex
- Id:
-
Dysgranular insular cortex
- Ig:
-
Granular insular cortex
- ils:
-
Inferior limiting sulcus
- LA:
-
Lateral auditory field
- LGN:
-
Lateral geniculate nucleus
- L:
-
Lateral nucleus of the amygdala
- los:
-
Lateral orbital sulcus
- mos:
-
Medial orbital sulcus
- OT:
-
Olfactory tubercle
- ot:
-
Optic tract
- ots:
-
Occipitotemporal sulcus
- PAC:
-
Periamygdaloid cortex
- PA:
-
Posterior auditory field
- Pi:
-
Parainsular cortex
- PIR:
-
Piriform cortex
- PL:
-
Paralaminar nucleus of the amygdala
- pmts:
-
Posterior middle temporal sulcus
- PPN:
-
Peripeduncular nucleus
- ps:
-
Principal sulcus
- RA:
-
Rostral auditory field
- RI:
-
Retroinsular cortex
- ros:
-
Rostral sulcus
- rs:
-
Rhinal sulcus
- sls:
-
Superior limiting sulcus
- SN:
-
Substantia nigra
- sts:
-
Superior temporal sulcus
- TA Area:
-
TA of Von Bonin and Bailey
- TE Area:
-
TE of Von Bonin and Bailey
- TEO Area:
-
TEO of Von Bonin and Bailey
- TF Area:
-
TF of Von Bonin and Bailey
- TH Area:
-
TH of Von Bonin and Bailey
- V:
-
Lateral ventricle
References
Aggleton JP, Burton MJ, Passingham RE (1980) Cortical and subcortical afferents to the amygdala of the rhesus monkey. Brain Res 190:347–368
Amaral DG, Bassett J (1989) Cholinergic innervation of the monkey amygdala: an immunohistochemical analysis with antisera to choline acetyltransferase. J Comp Neurol 281:337–361
Amaral DG, Price JL (1983) An air pressure system for the injection of tracer substances into the brain. J Neurosci Methods 9:35–43
Amaral DG, Veazy RB, Cowan WM (1982) Some observations on hypothalamo-amygdaloid connections in the monkey. Brain Res 252:13–27
Cowan WM, Gottlieb DI, Hendrickson AE, Price JL, Woolsey TA (1972) The autoradiographic demonstration of axonal connections in the central nervous system. Brain Res 37:21–55
Cuenod M, Streit P (1983) Neuronal tracing using retrograde migration of labeled transmitter-related compounds. In: Björklund A, Hökfelt T (eds) Methods in chemical neuroanatomy. Elsevier, Amsterdam The Netherlands, pp 365–397
Femano PA, Edinger HM, Siegel A (1979) Evidence of a potent excitatory influence from substantia innominata on basolateral amygdaloid units: a comparison with insulartemporal cortex and lateral olfactory tract stimulation. Brain Res 177:361–366
Fischer BO, Ottersen OP, Storm-Mathiesen J (1982) Labelling of amygdalopetal and amygdalofugal projections after intra-amygdaloid injections of tritiated D-aspartate. Neuroscience [Suppl]7:S69
Fonnum F, Sreide A, Kvale I, Walker J, Walaas I (1981) Glutamate in cortical fibers. In: Di Chiara G, Gessa GL (eds) Glutamate as a neurotransmitter. Raven Press, New York, pp 29–41
Fukuda AG, Ono T, Nakamura K (1987) Functional relations among inferotemporal cortex, amygdala, and lateral hypothalamus in monkey operant feeding behavior. J Neurophysiol 57:1060–1077
Herzog AG, Van Hoesen GW (1976) Temporal neocortical afferent connections to the amygdala in the rhesus monkey. Brain Res 115:57–69
Iwai E, Yukie M (1987) Amygdalofugal and amygdalopetal connections with modality-specific visual cortical areas in macaques (Macaca fuscata,M. mulatta, andM. fascicularis). J Comp Neurol 261:362–387
Iwai E, Yukie M, Suyama H, Shirakawa S (1987) Amygdalar connections with middle and inferior temporal gyri of the monkey. Neurosci Lett 83:25–29
Insausti R, Amaral DG, Cowan WM (1987) The entorhinal cortex of the monkey: II. Cortical afferents. J Comp Neurol 264:356–395
Jones EG, Burton H (1976) Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal and temporal regions of primates. J Comp Neurol 168:197–248
Jones EG, Burton H, Saper CB, Swanson LW (1976) Midbrain, diencephalic and cortical relationships of the basal nucleus of Meynert and associated structures in primates. J Comp Neurol 167:386–419
Kisvarday ZF, Cowey A, Smith AD, Somogyi P (1989) Interlaminar and lateral excitatory amino acid connections in the striate cortex of monkey. J Neurosci 9:667–682
LeVay S, Sherk H (1981) The visual claustrum. I. Structure and connections. J Neurosci 1:956–980
Mehler WR (1980) Subcortical afferent connections of the amygdala in the monkey. J Comp Neurol 190:733–762
Mesulam MM (1976) The blue reaction product in horseradish peroxidase histochemistry. Incubation parameters and visibility. J Histochem Cytochem 24:1273–1280
Norita M, Kawamura K (1980) Subcortical afferents to the monkey amygdala: an HRP study. Brain Res 190:225–230
Ottersen OP (1982) Connections of the amygdala of the rat. IV. Corticoamygdaloid and intra-amygdaloid connections as studied with axonal transport of horseradish peroxidase. J Comp Neurol 205:30–48
Pandya KN, Van Hoesen GW, Domesick VB (1973) A cingulo-amygdaloid projection in the rhesus monkey. Brain Res 61:369–373
Pitkänen A, Amaral DG (1991) Demonstration of projections from the lateral nucleus to the basal nucleus of the amygdala: a PHA-L study in the monkey. Exp Brain Res 83:465–470
Price JL, Russchen FT, Amaral DG (1987) The amygdaloid complex. In Björklund A, Hökfelt T, Swanson LE (eds) Handbook of chemical neuroanatomy, Vol 5. Integrated systems, Part I. Elsevier, Amsterdam, pp 279–388
Porrino LJ, Crane AM, Goldmann-Rakic PS (1981) Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkey. J Comp Neurol 198:121–136
Rosene DL, Roy NJ, Davis BJ (1986) A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J Histochem Cytochem 34:1301–1315
Russchen FT, Amaral DG, Price JL (1985) The afferent connections of the substantia innominata in the monkey, Macaca fascicularis. J Comp Neurol 242:1–27
Saunders RC, Rosene DL, Van Hoesen GW (1988) Comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey. II. Reciprocal and nonreciprocal connections. J Comp Neurol 271:185–207
Streit P (1981) Selective retrograde labeling indicating the transmitter of neuronal pathways. J Comp Neurol 191:429–463
Streit P (1984) Glutamate and aspartate as transmitter candidates for systems of the cerebral cortex. In: Jones EG, Peters A (eds) Cerebral cortex, Vol 2. Functional properties of cortical cells. Plenum Press, New York, pp 119–143
Szabo J, Cowan WM (1984) A stereotaxic atlas of the brain of the cynomolgus monkey(Macaca fascicularis). J Comp Neurol 222:265–300
Turner BH, Mishkin M, Knapp M (1980) Organization of the amygdalopetal projections from modality-specific cortical association areas in the monkey. J Comp Neurol 191:515–543
Van Hoesen GW (1981) The differential distribution, diversity and sprouting of cortical projections to the amygdala in the rhesus monkey. In: Ben-Ari Y (ed) The amygdaloid complex. Elsevier/North-Holland Biomedical Press, Amsterdam, pp 77–90
Von Bonin G, Bailey P (1947) The neocortex of macaca mulatta. University of Illinois Press, Urbana
Walker JE, Fonnum F (1983) Regional cortical glutamergic and aspartergic projections to the amygdala and thalamus of the rat. Brain Res 267:371–374
Whitlock DG, Nauta WJH (1956) Subcortical projections from the temporal neocortex in Macaca mulatta. J Comp Neurol 106:183–212
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Amaral, D.G., Insausti, R. Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex. Exp Brain Res 88, 375–388 (1992). https://doi.org/10.1007/BF02259113
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02259113