Abstract
Objective
Stress is known to increase cocaine craving and relapse risk in cocaine dependence. Identifying neural activity associated with stress and stress-induced cocaine craving is important in understanding the neurobiology of cocaine craving and relapse.
Method
Blood oxygenation level dependent (BOLD) signal changes were assessed in a functional magnetic resonance imaging (fMRI) session with healthy controls and treatment-engaged, abstinent, cocaine-dependent individuals (patients) as they participated in brief guided imagery and recall of three personal stress and three personal neutral situations.
Results
During stress, patients showed significantly less BOLD activation than controls in specific frontal and para-limbic regions, such as the anterior cingulate (AC) region, left hippocampal/parahippocampal region, right fusiform gyrus, and the right postcentral gyrus. On the other hand, patients had increased activity in the caudate and dorsal striatum region during stress, activation that was significantly associated with stress-induced cocaine craving ratings.
Conclusions
Patients failed to activate AC and related circuits during stress, regions associated with control, and regulation of emotion and distress states. Instead, they exhibited greater craving-related activation in the dorsal striatum, a region related to reward pathways and part of the obsessive–compulsive circuitry. Such functional alterations in stress processing may underlie the stress-related vulnerability to cocaine relapse often observed in cocaine-dependent individuals in early recovery.
Similar content being viewed by others
Introduction
Drug craving is a cardinal feature of cocaine dependence, and one that has been associated with the chronic relapsing nature of the disorder (Gavin and Kleber 1986; O'Brien et al. 1998). It increases with exposure to drug and drug-related cues, as well as in the context of emotional stress or negative mood (Childress et al. 1994; Jaffe et al. 1989; O'Brien et al. 1998; Sinha et al. 1999, 2000). Stress- and cue-related cocaine craving is accompanied by autonomic arousal and hypothalamic-pituitary adrenal (HPA) activation (Sinha et al. 2003) and has been shown to predict relapse after inpatient drug treatment (Cooney et al. 1997; Sinha et al. 2004a). These findings are consistent with clinical observations that cocaine-dependent individuals have difficulties managing stressful life events, which often increases their susceptibility to relapse (McKay et al. 1995; O'Brien et al. 1998; Sinha 2001). Thus, a better understanding of the neural activity associated with stress and stress-induced drug craving could be useful in developing treatments to prevent relapse to cocaine dependence.
Brain imaging studies of cocaine-related drug craving (drug priming) and of cocaine-cue-related drug craving show increased activity in stress and emotion processing-related brain regions as well as in reward-related pathways (Breiter et al. 1997; Childress et al. 1999; Grant et al. 1996; Kilts et al. 2001; Maas et al. 1998; Wexler et al. 2001). For example, cocaine-induced drug craving activates the ventral striatum (Breiter et al. 1997) and other fronto-limbic regions, whereas cocaine-cue-induced craving is associated with activation in the anterior cingulate (AC), amygdala, nucleus accumbens, and the dorsolateral prefrontal cortex (Childress et al. 1999; Grant et al. 1996; Kilts et al. 2001; Maas et al. 1998; Wexler et al. 2001). Similarly, emotional distress and aversive stimulus exposure also activates cortico-limbic circuits, including prefrontal, anterior cingulate, middle frontal, and orbitofrontal regions, and limbic and paralimbic regions such as the amygdala, hippocampus, parahippocampal gyrus, fusiform gyrus, and other midbrain regions, but not the ventral striatum (Sinha et al. 2004b; Li et al. 2005; see Phan et al. 2002 for review).
As stress-related coping difficulties are often associated with cocaine dependence, we hypothesized that brain responses to stress would differ between cocaine abusers and healthy controls. We also predicted that neural activity during stress-induced cocaine craving would involve frontal–limbic–striatal circuitry associated with both emotion and reward processing. Stress was induced using script-based guided imagery procedures, a method commonly used to induce negative emotions, stress, and drug craving in healthy and psychiatric populations (Drobes and Tiffany 1997; Kilts et al. 2001; Sinha et al. 1999, 2000, 2003).
Subjects and methods
Subjects
Individuals between the ages of 21 and 50 participated as study volunteers. Twenty cocaine-dependent individuals (16 men and 4 women) who were residing on a locked inpatient treatment research facility volunteered to participate in this study. All patients were participating in treatment for their cocaine addiction and had been substance free for a minimum of 2 weeks prior to the brain imaging session. They met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for current cocaine dependence as diagnosed by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (First et al. 1995), with self reports of three or more times of weekly cocaine use, documented by positive urine toxicology screens prior to inpatient admission. Individuals who met current DSM-IV criteria for dependence on another psychoactive substance other than alcohol and nicotine were excluded. In addition, those who were currently on medications for medical or psychiatric problems and those in need of alcohol detoxification were excluded from the study. In addition to the cocaine patients, eight healthy control individuals (seven men and one woman) with no history of drug abuse and who were not currently on any medications were recruited through advertising and flyers posted in local area newspapers. All subjects underwent a complete medical evaluation, including electrocardiography and laboratory tests of renal, hepatic, pancreatic, hematopoietic, and thyroid functions to ensure good physical health. The study procedures were approved by the Human Investigation Committee at the Yale University School of Medicine, and all subjects signed an informed consent prior to study participation.
Methods
Imagery script development session
In the week prior to the functional magnetic resonance imaging (fMRI) session, scripts for the guided imagery induction were developed in a structured clinical interview session. Three stress imagery scripts were developed for each subject. They were based on the subjects' description and ratings of three separate personal, stressful events that were experienced as “most stressful” within the past year. “Most stressful” was determined by having each subject rate their level of emotional distress experienced on a 10-point Likert scale, where “1=not at all distressing” and “10=the most distress they felt recently in their life.” Only situations rated as 8 or above on this scale were accepted as appropriate for script development. In addition to the stress scripts, three neutral-relaxing scripts were also developed from personalized, neutral, drug-free situations. Examples of acceptable stressful situations include breakup with significant other, a verbal argument with a significant other or family member, or unemployment-related stress, such as being fired or laid off from work. Examples of neutral relaxing situations included are a summer beach scene, relaxing Sunday afternoon reading, and/or a fall day at the park.
A “script” or description of each stress and neutral situation was developed using Scene Development Questionnaires (SDQ) and methods described previously (Miller et al. 1987; Sinha et al. 1992 ). Briefly, the SDQ obtains specific details on the physical, interpersonal, verbal/cognitive context, and bodily responses experienced for each situation. On the basis of these details, three stress and three neutral scripts of 2 min in length were developed for each subject and recorded on an audiotape for the fMRI session. The order of the stress and neutral scripts was assigned randomly.
To reduce variability in imagery ability and to train all subjects in progressive relaxation, a one-session progressive relaxation and guided imagery training session was conducted with all subjects according to procedures outlined by Miller et al. (1987) and used previously in our imagery studies with controls and cocaine abusers (Sinha et al. 1992, 2003).
fMRI image acquisition procedures
Images were obtained using a 1.5-T MRI system (GE Medical Systems, Milwaukee, WI, USA) equipped with a standard quadrature head-coil and echoplanar capability. Head positioning was standardized using the canthomeatal line and was secured with foam pillows and a band across the forehead. Subjects wore headphones (Resonance Technology, CA, USA) and were fitted with a pulse-oximeter on their finger to obtain heart rate, recorded every 10 s. Conventional T1-weighted spin-echo sagittal anatomic images (TR 500 ms, minimum TE, 102×256, 22 slices, slice thickness 5 mm, no slice gap, and field of view of 20) were acquired for slice localization. Anatomic T1-weighted spin-echo images in the coronal plane perpendicular to the AC–PC line (TR=500 ms, TE=15 ms, slice thickness 6 mm, and a 1-mm slice gap, 192×256 data matrix, with a field of view of 20, 2 Nex) were acquired to serve as underlays for functional images. Functional, blood oxygen level dependent (BOLD) signals using a single-shot, echoplanar-gradient echo sequence (17 coronal slices perpendicular to the AC–PC line and starting at the frontal poles, TR 1.5 sec, TE 45 ms, flip angle 85°, 64×64 data matrix, field of view 20 cm, slice thickness 6 mm, gap 1 mm, 220 images per slice) were then obtained.
fMRI imaging trials
Six functional imaging trials (three stress and three neutral script runs) were acquired, lasting 5.5 min each. Each 5.5-min trial included a 1.5-min quiet baseline (B) period, followed by a total 2.5-min guided imagery (I) period that included a 2-min read-image period and a 30-s quiet-image period, followed by a 1-min quiet post-imagery recovery period. Before and after the end of each scanning trial, subjects verbally rated their level of subjective distress to the question “how stressed or anxious are you feeling right now” on an auditory analog Likert scale ranging from 0–10. Patients were also asked to rate their desire for cocaine (cocaine craving) on an auditory analog Likert scale ranging from 0–10. After each trial, subjects also rated the imagery vividness on a 10-point scale. There were no differences between groups or in stress and neutral conditions on imagery vividness (stress trials, cocaine patients=7.43, SD=3.07, controls=8.06, SD=1.8; neutral trials, cocaine patients=7.05, SD=2.1, controls=7.15, SD=1.6). Between imaging trials, subjects participated in progressive relaxation for 2 min to help reduce any leftover anxiety or distress from the previous trial. Subsequent trials were not initiated until the subjects' ratings of anxiety and pulse rate were stabilized down to their baseline levels. Using this procedure, there were no differences in baseline anxiety and pulse across trials. Furthermore, all subjects had been trained in progressive relaxation and guided imagery using a single session progressive relaxation and guided imagery training procedure developed by Lang and colleagues (Miller et al. 1987) that has been used in our previous studies (Sinha et al. 1992, 1999, 2000) and described in detail previously (Sinha et al. 2003). We have found such training to be helpful in reducing baseline stress levels between imagery trials in single session experiments (Sinha et al. 1999, 2000).
fMRI data analysis
Images were first motion corrected for three translational directions and three possible rotations (Friston et al. 1996). Trials with linear motion that had a displacement in excess of 1.5 mm or rotation in excess of 2° were rejected. For the 20 cocaine patients, 5 neutral trials were removed, and 1 subject only completed 2 trials leaving 54 total neutral trials in the patients. Similarly, 2 stress trials were removed due to motion and one subject only completed 2 trials leaving 57 total stress trials in the patient group. For the eight controls, 2 neutral trials were removed due to motion leaving 22 total neutral trials, and 4 stress trials were removed due to motion leaving 20 total stress trials. The corrected images were spatially filtered using a Gaussian filter with a full width at half maximum of 6.25 mm.
Data were analyzed using MATLAB and the Yale fMRI analysis package (Skudlarski et al. 1999) used extensively in previous studies (Wexler et al. 2001), (Blumberg et al. 2003; Potenza et al. 2003; Shaywitz et al. 1999). For each subject, separate percent signal change maps of imagery (I) period relative to the initial baseline (B) period were created for the stress and the neutral trials. Images for each subject were transformed into common stereotactic space by piece-wise linear warping. (Friston et al. 1995; Talairach and Tournoux 1988). A standard reslicing of the Talairach space where the whole brain is cut into 19 slices (8 anterior to AC, 3 between AC and PC, and 8 posterior to PC) was used. As this study did not cover the whole brain, only 14 of the 19 Talairach slices were obtained.
The percent signal change maps from individual subjects were used as a derived measure of task-related activity. The statistical significance was assessed only for the composite maps (within each group and between groups) using a randomization procedure with nonparametric statistics. To avoid the need to assume a specific distribution and variance of the data, a randomization procedure was used to estimate p values of the group composite maps (Manly 1997). To randomize, the sign of the activation measure for each voxel, which is the mean t value, was reversed in randomly generated subsets of subjects. The activation measure was then recalculated. This procedure was repeated 1,000 times, generating a distribution of the activation measure. The proportion of times that the observed activation measure was more extreme than a randomized value represents a p value. It is the proportion of times we would expect to obtain a mean activation as large or larger than the one obtained if the null hypothesis (no difference between tasks) were true. The p value at each voxel (p<0.05) was then overlaid on the mean anatomic image for display (Shaywitz et al. 1995; Skudlarski et al. 1999; Wexler et al. 2001). It is important to note that both the task-related activation maps (derived from images in the I-B periods) and the randomization procedures (used to test statistical significance for the within-group and the between-group differences at each voxel location) do not require equal sample sizes, and thus, the significance estimates are calculated using the sample size of both groups (Manly 1997; Skudlarski et al. 1999).
There are two possible ways that the group comparisons can be conducted. First, each subject's stress difference map (I-B) could be subtracted from the neutral difference map (S-N), and the dual change maps can be contrasted once again to examine group differences. Alternatively, each subject's difference map for each condition can be contrasted to examine group differences, i.e., both the stress and the neutral condition maps are contrasted separately across groups. We selected the latter approach to limit the number of data subtractions/contrasts that were being conducted which can lead to greater difficulties in interpretation of the data. Furthermore, as the neutral stimuli used in the current study are relaxing and therefore somewhat pleasant, a direct contrast between stress and neutral trials could also result in greater difficulty in interpretation of data. Instead, comparing groups separately for stress and neutral trials provided a more straightforward approach to understanding the findings.
The data analysis procedures described above were procedures used for both the within-group comparisons (change from baseline in each group per condition) and the between-group comparisons (patients vs controls). As in our previous brain imaging studies, within-group comparisons were presented at a significance threshold of p<0.001 with the additional application of a minimum 20-voxel extent cluster filter. Combining the significance threshold with a cluster filter is a conservative and yet efficient way to examine the data (Forman et al. 1995). The between-group difference significance level was set at p<0.01 with a minimum 20-voxel extent cluster filter. Talairach (x, y, z) coordinates corresponding to activity change peaks were determined using the Yale fMRI analysis package (Skudlarski et al. 1999). Finally, voxel-based (whole brain) correlations were conducted to examine associations between brain activity regions and ratings of subjective distress and cocaine craving. The significance threshold for the voxel-based whole brain correlation analysis was set at p<0.005 (uncorrected, extent threshold of 20 voxels).
Results
Baseline characteristics
Healthy controls were younger than the patients (controls mean age 32.8, SD=4.74; patients mean age 38.75, SD=4.77; t value 3.12, p<0.004) and both groups were equivalent on years of education (controls 13.44, SD=2.06; patients 12.6, SD=1.29, p=NS). There were no differences between groups on race [controls, 55% Caucasian, 33% African American (AA); patients, 35% Caucasian, 60% AA], left-handedness (controls, 1/8 or 12.5%; patients, 3/20 or 15%), lifetime history of major depression (controls, 2/8 or 25%; patients, 6/20 or 33%), and lifetime history of any anxiety disorders (controls, 3/8 or 37.5%; patients, 5/20 or 25%). Cocaine-dependent subjects reported regular cocaine use for a period of 9 years (SD=6) with average cocaine use on 14 days (SD=9.08) and spending an average of \$224 per week (SD=\$335) (approximately 2 g of crack weekly) in the 30 days prior to inpatient admission. Patients reported smoking cigarettes daily and used alcohol on 10.7 days (SD=22) of the past 30, with an average consumption of nine drinks per week (SD=12.3) prior to admission. Thirty percent (6/20) met DSM-IV criteria for current alcohol dependence, and 10% (2/20) met DSM-IV criteria for current marijuana dependence. Controls were light social drinkers with an average consumption of 1.3 drinks per week (SD=2.13), and only one control subject reported smoking cigarettes (7/day). No other drug use was reported among controls.
Subjective and physiological responses during fMRI trials
Both controls and patients reported significantly greater subjective distress during stress than in the neutral trials [F(1,26)=65.77, p<0.0001], with no significant differences between groups (see Fig. 1a). Significant increases in average heart rate was observed during stress as compared to neutral trials [F(1,24)=36.7, p<0.0001] with no differences between groups (Fig. 1b). Cocaine subjects also reported significantly greater cocaine craving during stress than in neutral trials (t=5.15, p<0.0001) (see Fig. 1c).
fMRI signal changes during stress and neutral trials
As shown in Tables 1a and b, controls and patients showed increases in fMRI BOLD signal bilaterally in the primary auditory cortex [R and L superior temporal cortex, Brodmann area (BA) 22], the middle frontal region (BA 9, 45), and left inferior frontal region (BA 45, 47) during both stress and neutral trials with no significant differences in level of activation between groups.
Multiple additional areas showed signal changes during stress, but not the neutral trials in both patients and controls in the within-group analyses (Table 1a lists the Talairach coordinates based on the average center of mass for activity change at a given y level). Bilateral activation in the thalamus/putamen, caudate, the posterior cingulate (PC) (BA 23), and the superior frontal gyrus (BA 8) was observed in both groups. Furthermore, only patients showed a significant deactivation in the medial orbitofrontal/anterior cingulate region (BA 32/11), whereas only controls showed significant activation of the medial prefrontal/anterior cingulate gyrus (BA 6/32).
Between-group differences between controls (C) and patients (P) during stress were observed for several other cortical–paralimbic regions (see Fig. 2, column C, P-C contrasts at p<0.01 shown in blue/purple). Controls showed significantly increased activation of the anterior cingulate region (BA 32, 6) as compared to patients (Fig. 2, column C, y=4 mm), resulting from increased activity in the AC in controls, but decreased activity in the AC in patients (see Table 1a). Furthermore, the deactivation in the medial frontal region (BA 6) extended dorsally in patients resulting in a significant difference between controls and patients for the lateral frontal cortex (see Fig. 2, column C, y=−20 mm). A similar group difference was seen for the hippocampus and parahippocampal region with controls showing increased activity during stress and no significant activation in cocaine patients (y=−28 mm). The right fusiform gyrus also showed a significantly greater activation in controls as compared to the cocaine patient group (Fig. 2, column C, y=−8 mm). Finally, significant differences between groups in the R and L postcentral gyrus (BA 2) were seen (Fig. 2, column C, y=−20 and −28 mm), arising only from patients showing a deactivation in these regions in the stress trials, and only in the R postcentral gyrus during the neutral trials (see Table 1b).
fMRI images representing BOLD signal changes during the stress image period relative to initial baseline (p<0.001, uncorrected) in a patients (P), b healthy controls (C), and in c patients relative to controls (P–C, p<0.01); AC anterior cingulate cortex, LPreCG left precentral gyrus, RFG right fusiform gyrus, RPostCG right postcentral gyrus, MdFG medial frontal gyrus, LH left hippocampus
Finally, patients showed increased activity bilaterally relative to baseline in the dorsal striatum/caudate, a region that did not show greater activity at the p<0.001 threshold in controls (see Table 1a, and Fig. 2, column A and B, y=4 and −4 mm). However, this differential increase from baseline in the cocaine group was not significantly different than controls at the p<0.01 level (column C).
Brain regions associated with subjective distress and cocaine craving (p<0.005)
Results of the voxel-based whole brain correlation analysis are presented in Table 2 (p<0.005, uncorrected; extent threshold of 20 voxels). Controls showed significant positive associations between distress ratings and BOLD activity in the right caudate/basal ganglia and thalamic region, left fusiform gyrus, and the left hippocampal/parahippocampal region. A significant inverse association between distress ratings and the anterior cingulate region and the right insula was also observed in controls.
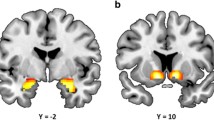
Patients showed significant positive associations between right dorsolateral prefrontal region (BA 9, 46) and both distress and cocaine craving. The left posterior insula/superior temporal gyrus (STG; BA 22) was also correlated positively with both distress and with cocaine craving. In addition, subjective distress ratings correlated positively with the posterior cingulate (BA 23). Finally, cocaine craving was significantly associated with the right caudate and the R dorsomedial thalamus (see Fig. 3a,b) regions that showed increased activity relative to baseline in patients (see Fig. 2 and Table 1a).
Voxel-based correlation maps in patients of the significant association between BOLD signal change in the a right caudate (RCaud), b right striatal/thalamic region (RThal/Str), and left insula/superior temporal gyrus (L ins/STG) and increase in stress-induced cocaine craving ratings (for specific coordinates, see Table 2)
Discussion
This fMRI BOLD activation study of stress and stress-induced cocaine craving shows altered brain responses to stress in cocaine patients compared to controls. During stress, controls showed significantly greater activation than cocaine-dependent individuals in frontal–paralimbic areas including the anterior cingulate (AC), left hippocampal/parahippocampal gyrus, and the R fusiform gyrus. Significant deactivation was observed in the right postcentral gyrus during stress in cocaine patients as compared to controls. In contrast, patients showed increased bilateral activation in the dorsal striatum and caudate region during stress, with the right-side activity showing a significant positive correlation with stress-induced cocaine craving.
Decreased brain activity in the anterior cingulate and associated paralimbic regions in cocaine patients may suggest an inability to exercise “willful” control of behavior in the face of stressful situations (Goldstein and Volkow 2002; Peoples 2002). Anterior cingulate and related circuits are thought to be involved in emotion regulation (Beauregard et al. 2001; Critchley et al. 2001; Lane et al. 1998), and increases in AC activity are also seen with mental stress challenge (Soufer et al. 1998) and during tasks of cognitive control and response inhibition (Carter et al. 1998; Garavan et al. 2002). In a recent study, Kaufman et al. (2003) reported hypoactivity in the AC in cocaine abusers compared to controls during a GO–NOGO task that requires cognitive control and inhibition of prepotent responding. The current results are consistent with this previous research in nondrug using subjects showing significant increases in anterior cingulate activity during stress (Soufer et al. 1998). The AC activity was also negatively associated with distress ratings (see Table 2), suggesting a stress regulatory function of this region among controls in the current study. On the other hand, cocaine abusers showed hypoactivity of the AC during stress relative to controls. Previous research has documented abnormalities in medial prefrontal, orbitofrontal, and anterior cingulate regions in the brains of cocaine addicts (Franklin et al. 2002; Goldstein and Volkow 2002), and alterations in dopamine-related frontal–striatal pathways has also been noted in nonhuman primates chronically exposed to cocaine (Porrino and Lyons 2000). Whereas the reported differences between patients and controls in the current study could be related to premorbid differences between groups in stress-related processing, cocaine-related alterations in anterior cingulate circuitry could also compromise the functional integrity of these regions. Such “hypofunctionality” in the anterior cingulate and related circuits may explain cocaine-dependent individuals' difficulties in managing distress and in making adaptive decisions regarding future actions in the face of stress (Goldstein and Volkow 2002; Sinha 2001).
In contrast, increased activation of the dorsal striatum/caudate region in patients was associated with stress-induced drug craving (see Fig. 3). Whereas the ventral striatum is critically involved in drug reinforcement, the dorsal striatum has been associated with learned habits and skill-based procedural learning (Berke and Hyman 2000; Jog et al. 1999). Thus, the stress-induced cocaine-craving-related dorsal striatum/caudate activity could represent the automatized, well-learned, habit-based nature of craving and drug use behaviors in addiction (Berke and Hyman 2000; Tiffany 1990). This increase in craving-related dorsal striatum/caudate activity combined with the decreased activity in anterior cingulate circuits suggests a neural response involving decreased emotion regulation and control and increases in habits/compulsions during stress. Such a response pattern may underlie the stress-related susceptibility to cocaine seeking and relapse observed in cocaine patients.
Behavioral ratings of distress and heart rate increases did not differ between controls and patients, suggesting that both groups showed equivalent levels of subjective distress and physiological arousal. Furthermore, several brain regions showed similar increases in activity during stress in both groups. For example, increased activation in the superior frontal gyrus (BA 8), posterior cingulate, thalamus, and putamen was observed in both groups (see Table 1a and b). Several previous neuroimaging studies have shown increased activity in these cortico-limbic regions during various mood and emotion manipulation studies (see Phan et al. 2002 for a comprehensive review). Activity in these regions suggests a role for these areas in affective processing, particularly in processing aversive, anxious, and negative mood-related stimuli (Phan et al. 2002). These findings add further credence to the experimental manipulation in that both groups appeared to be responding in similar ways to the experience and processing of distress, but showed specific differences in brain regions involved in emotion regulation and cognitive control.
In addition to the caudate and thalamic regions, significant positive correlations were found for the right dorsolateral prefrontal (DLPFC) region (BA 9, 46) and subjective distress as well as cocaine craving (see Table 2). The DLPFC has been associated with higher executive processing including recall of events from memory, and the right prefrontal regions are known to be involved in the experience of negative emotions (Fuster 2001). The DLPFC correlations with both distress as well as stress-induced craving in this study suggest its involvement both in the reexperiencing of distress as well as in recall of behavioral intention to use drugs in patients. Subjective distress ratings were also correlated with the posterior cingulate and the left insula (posterior section)/STG region in patients, with the latter region also showing an association with cocaine craving (see Table 2). Both the posterior cingulate and the insula are part of the limbic circuitry known to play a role in emotion processing and affect regulation (Davidson et al. 2000; Devinsky et al. 1995; Phan et al. 2002; Sinha et al. 2004b), whereas the posterior cingulate and the STG are also involved in the retrieval of contextual and affect-laden autobiographical information (Fink et al. 1996; Fujii et al. 2004; Maddock et al. 2001; Piefke et al. 2003). Positive associations with the posterior cingulate and the left posterior insula/STG in the current study support their involvement in experiencing distress, and, in the case of the left insula/STG, its role in the urge/action component of the stress experiences in patients. On the other hand, controls showed a negative association between distress and the right anterior insula, suggesting that the right anterior insula plays a role in modulating distress in controls.
Limitations of the study include a small sample of control subjects, and that gender differences were not studied due to the few women in the sample. Clearly, the current findings need replication with more subjects, and given that sex differences in stress- and cue-induced brain activation in cocaine abusers has recently been documented (Kilts et al. 2004; Li et al. 2005), an examination of gender differences between controls and cocaine abusers is warranted. It is also important to note that certain areas such as the amygdala that is known to be involved in emotional processing was not found to be active during this study. However, the brain imaging parameters were not necessarily selected to detect activations in high susceptibility regions such as in the amygdale, and it is possible that with a lower TE and thinner slices, such activity could have been detected. Both specific imaging parameters and small sample size may contribute to an inability to detect all possible brain areas that could be involved in emotional stress processing. Finally, cocaine patients in the current study were abstinent, treatment-engaged individuals, and therefore, these results may not extend to nontreatment-seeking cocaine abusers.
Despite the above limitations, this is one of the first studies that employed functional brain imaging to document altered brain responses to stress exposure in cocaine patients compared to controls, with hypofunction in the anterior cingulate and related circuits and increased activity of the dorsal striatum/caudate region that was correlated with stress-related cocaine craving. This pattern of neural activity may underlie stress-related vulnerability to cocaine relapse and could prove to be a useful biological marker in assessing new treatments that target attenuation of stress-induced cocaine craving as a relapse precipitant.
References
Beauregard M, Levesque J, Bourgouin P (2001) Neural correlates of conscious self-regulation of emotion. J Neurosci 21:1–6
Berke JD, Hyman SE (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25:515–532
Blumberg HP, Leung H, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, Charney DS, Gore JC, Krystal JH, Peterson BS (2003) A functional magnetic resonance imaging study of bipolar disorder. Arch Gen Psychiatry 60:601–609
Breiter HC, Gollub RL, Weiskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL (1997) Acute effects of cocaine on human brain activity and emotion. Neuron 19:591–611
Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998) Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280:747–749
Childress AR, Ehrman RN, McLellan AT, MacRae J, Natale M, O'Brien CP (1994) Can induced moods trigger drug-related responses in opiate abuse patients? J Subst Abuse Treat 11:17–23
Childress AR, Mozley PD, McElgin W, Fitzgerald J, Revich M, O'Brien CP (1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156:11–18
Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L (1997) Alcohol cue reactivity, negative mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychology 106:243–250
Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ (2001) Brain activity during biofeedback relaxation: a functional neuroimaging investigation. Brain 124:1003–1012
Davidson RJ, Putnam KM, Larson CL (2000) Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science 289:591–594
Devinsky O, Morrell MJ, Vogt BA (1995) Contributions of anterior cingulate cortex to behaviour. Brain 118:279–306
Drobes DJ, Tiffany ST (1997) Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. J Abnorm Psychology 106:15–25
Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD (1996) Cerebral representation of one's own past: neural networks involved in autobiographical memory. J Neurosci 16:4275–4282
First M, Gibbon M, Spitzer R, Williams J (1995) User's guide to the structured clinical interview for DSM-IV axis disorders (SCID-I, Version 2.0)
Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995) Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of cluster-size threshold. Magn Reson Med 33:636–647
Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O'Brien CP, Childress AR (2002) Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Soc Biol Psychiatry 51:134–142
Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ (1995) Spatial registration and normalization of images. Hum Brain Mapp 3:165–189
Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996) Movement-related effects in fMRI time-series. Magn Reson Med 35:346–355
Fujii T, Suzuki M, Okuda J, Ohtake H, Tanji K, Yamaguchi K et al (2004) Neural correlates of context memory with real-world events. Neuroimage 21:1596–1603
Fuster JM (2001) The prefrontal cortex—an update: time is of the essence. Neuron 30:319–333
Garavan H, Ross TJ, Murphy K, Roche RAP, Stein EA (2002) Dissociable executive functions in the dynamic control of behavior: inhibition, error detection and correction. Neuroimage 17:1820–1829
Gavin FH, Kleber HD (1986) Abstinence symptomatology and psychiatric diagnosis in cocaine abusers: clinical observations. Arch Gen Psychiatry 43:107–113
Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652
Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A (1996) Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A 93:12040–12045
Jaffe JH, Cascella NG, Kumor KM, Sherer MA (1989) Cocaine-induced cocaine craving. Psychopharmacology 97:59–64
Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM (1999) Building neural representations of habits. Science 286:1745–1749
Kaufman JN, Ross TJ, Stein EA, Garavan H (2003) Cingulate hypoactivity in cocaine users during a GO–NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci 23921:7839–7843
Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KPG (2001) Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 58:334–341
Kilts CD, Gross RE, Ely TD, Drexler KP (2004) The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry 161:233–241
Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE (1998) Neural correlates of levels of emotional awareness: evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci 10:525–535
Li C-S, Kosten TR, Sinha R (2005) Sex differences in brain response to stress imagery in abstinent cocaine dependent individuals—an fMRI study. Biol Psychiatry 57:487–494
Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF (1998) Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry 155:124–126
Maddock RJ, Garrett AS, Buonocore MH (2001) Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104:667–676
Manly B (1997) Randomization, Bootstrap and Monte Carlo methods in biology. Chapman and Hall, London
McKay JR, Rutherford MJ, Alterman AI, Cacciola JS, Kaplan MR (1995) An examination of the cocaine relapse process. Drug Alcohol Depend 38:35–43
Miller GA, Levin DN, Kozak MJ, Cook EW III, McLean A Jr, Lang PJ (1987) Individual differences in imagery and the psychophysiology of emotion. Cogn Emot 1:367–390
O'Brien CP, Childress AR, Ehrman RN, Robbins SJ (1998) Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 12:15–22
Peoples LL (2002) Will, anterior cingulate cortex, and addiction. Science 296:1623–1624
Phan KL, Wager T, Taylor SF, Liberzon I (2002) Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16:331–348
Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR (2003) Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain 126:650–668
Porrino LJ, Lyons D (2000) Orbital and medial prefrontal cortex and psychostimulant abuse: studies in animal models. Cereb Cortex 10:326–333
Potenza MN, Steinberg MA, Skudlarski P, Fulbright RK, Lacadie C, Wilber MK, Rounsaville BJ, Gore JC, Wexler BE (2003) Gambling urges in pathological gambling. Arch Gen Psychiatry 60:828–836
Shaywitz BA, Pugh KR, Constable RT, Shaywitz SE, Bronen RA, Fulbright RK, Shankweiler DP, Katz L, Fletcher JM, Skudlarski P, Gore JC (1995) Localization of semantic processing using functional magnetic resonance imaging. Hum Brain Mapp 2:149–158
Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Naftolin F, Palter P, Fletcher JM, Marchione KE, Katz L, Lacadie C, Keltz M, Gore JC (1999) Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. J Am Med Assoc 281:1197–1202
Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology 158:343–359
Sinha R, Lovallo WR, Parsons OA (1992) Cardiovascular differentiation of emotions. Psychosom Med 54:422–435
Sinha R, Catapano D, O'Malley SS (1999) Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology 142:343–351
Sinha R, Fuse T, Aubin LR, O'Malley SS (2000) Psychological stress, drug-related cues and cocaine craving. Psychopharmacology 152:140–148
Sinha R, Talih M, Malison R, Anderson GA, Cooney N, Kreek M (2003) Hypothalamic–pituitary–adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology 170:62–72
Sinha R, Garcia M, Malison RM, Kreek MJ (2004a) Stress induced cocaine craving and HPA responses predict cocaine relapse outcomes. Presented at the Annual Meetings of the American College of Neuropsychopharmacology, San Juan, PR, Dec 11–17
Sinha R, Lacadie C, Skudlarski P, Wexler BE (2004b) Neural circuits underlying emotional distress in humans. Ann N Y Acad Sci 1032:254–257
Skudlarski P, Constable RT, Gore JC (1999) ROC analysis of statistical methods used in functional MRI: individual subjects. Neuroimage 9:311–329
Soufer R, Bremner JD, Arrighi JA et al (1998) Cerebral cortical hyperactivation in response to mental stress in patients with coronary artery disease. Proc Natl Acad Sci U S A 95(11):6454–6459
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Thieme Medical Publications, New York
Tiffany ST (1990) A cognitive model of drug urges and drug use behavior: role of automatic and nonautomatic processes. Psychol Rev 97:147–168
Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC (2001) Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry 158:86–95
Acknowledgements
This research was supported by the National Institutes of Health grants R01-DA11077 (RS), P50-DA09241 (BJR), P50-DA16556 (RS), K05-DA0454 (TRK), K02-DA17232 (RS), and K02-MH01296 (BEW). This study was presented in part at the 42nd Annual Meetings of the American College of Neuropsychopharmacology, San Juan, Puerto Rico, Dec 11–17. We would like to thank Hedy Sarofin, Terry Hickey, and Chekeyma Prince for their technical assistance and Dr. John Gore and Dr. R Todd Constable for their support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sinha, R., Lacadie, C., Skudlarski, P. et al. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology 183, 171–180 (2005). https://doi.org/10.1007/s00213-005-0147-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0147-8