Abstract
Rationale
There is evidence that lesions of the nucleus accumbens core (AcbC) promote preference for smaller earlier reinforcers over larger delayed reinforcers in inter-temporal choice paradigms. It is not known whether this reflects an effect of the lesion on the rate of delay discounting, on sensitivity to reinforcer magnitude, or both.
Aim
We examined the effect of AcbC lesions on inter-temporal choice using a quantitative method that allows effects on delay discounting to be distinguished from effects on sensitivity to reinforcer size.
Materials and methods
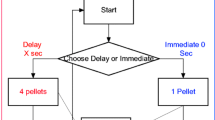
Sixteen rats received bilateral quinolinic acid-induced lesions of the AcbC; 14 received sham lesions. They were trained under a discrete-trials progressive delay schedule to press two levers (A and B) for a sucrose solution. Responses on A delivered 50 μl of the solution after a delay d A; responses on B delivered 100 μl after d B. d B increased across blocks of trials, while d A was manipulated across phases of the experiment. Indifference delay d B(50) (value of d B corresponding to 50% choice of B) was estimated in each phase, and linear indifference functions (d B(50) vs d A) derived.
Results
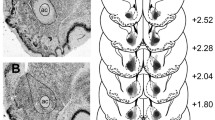
dB(50) increased linearly with dA (r2 > 0.95 in each group). The intercept of the indifference function was lower in the lesioned than the sham-lesioned group; slope did not differ between groups. The lesioned rats had extensive neuronal loss in the AcbC.
Conclusions
The results confirm that lesions of the AcbC promote preference for smaller, earlier reinforcers and suggest that this reflects an effect of the lesion on the rate of delay discounting.








Similar content being viewed by others
References
Acheson A, Farrar AM, Patak M, Hausknecht KA, Kieres AK, Choi S, de Wit H, Richards JB (2006) Nucleus accumbens lesions decrease sensitivity to rapid changes in the delay to reinforcement. Behav Brain Res 173:217–228
Ainslie G (1975) Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull 82:463–496
Bizot J, Le Bihan C, Puech AJ, Hamon M, Thiebot M (1999) Serotonin and tolerance to delay of reward in rats. Psychopharmacology 146:400–412
Bowman EM, Brown VJ (1998) Effects of excitotoxic lesions of the rat ventral striatum on the perception of reward cost. Exp Brain Res 123:439–448
Bradshaw CM, Body S, Szabadi E (2007) Decision-making and neuroeconomics: delayed reinforcement, neuroscience. In: Squire L (ed) New encyclopedia of neuroscience, MS#1527. Elsevier, Oxford, (in press)
Cardinal RN (2006) Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw 19:1277–1301
Cardinal RN, Robbins TW, Everitt BJ (2000) The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology 152:362–375
Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ (2001) Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292:2499–2501
Cardinal RN, Robbins TW, Everitt BJ (2003) Choosing delayed rewards: perspectives from learning theory, neurochemistry, and neuroanatomy. In: Heather N, Vuchinich RE (eds) Choice, behavioral economics and addiction. Elsevier, Oxford, pp 183–213
Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ (2004) Limbic corticostriatal systems and delayed reinforcement. Ann N Y Acad Sci 1021:33–50
Cheung THC, Cardinal RN (2005) Hippocampal lesions facilitate instrumental learning with delayed reinforcement but induce impulsice choice in rats. BMC Neurosci 6:36
Evenden JL, Ryan CN (1996) The pharmacology of impulsive behaviour in rats: The effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology 128:161–170
Evenden JL, Ryan CN (1999) The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology 146:413–421
Gibbon J (1977) Scalar expectancy theory and Weber’s law in animal timing. Psychol Rev 84:279–325
Gibbon J (1991) Origins of scalar timing. Learn Motiv 22:3–38
Green L, Fisher EB, Perlow S, Sherman L (1981) Preference reversal and self-control—choice as a function of reward amount and delay. Behav Anal Lett 1:43–51
Herrnstein R (1981) Self-control as response strength. In: Bradshaw CM, Szabadi E, Lowe CF (eds) Quantification of steady-state operant behaviour. Elsevier, Amsterdam, pp 3–20
Ho MY, Mobini S, Chiang TJ, Bradshaw CM, Szabadi E (1999) Theory and method in the quantitative analysis of “impulsive choice” behaviour: implications for psychopharmacology. Psychopharmacology 146:362–372
Ho M-Y, Velazquez-Martinez DN, Bradshaw CM, Szabadi E (2002) 5-Hydroxytryptamine and interval timing behaviour. Pharmacol Biochem Behav 71:773–785
Jongen-Relo AL, Feldon J (2002) Specific neuronal protein: A new tool for histological evaluation of excitotoxic lesions. Physiol Behav 76:449–456
Kheramin S, Body S, Mobini S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM (2002) Effects of quinolinic acid-induced lesions of the orbital prefrontal cortex on inter-temporal choice: a quantitative analysis. Psychopharmacology 165:9–17
Kheramin S, Body S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM (2003) Role of the orbital prefrontal cortex in choice between delayed and uncertain reinforcers: a quantitative analysis. Behav Processes 64:239–250
Kheramin S, Body S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM (2004) Effects of orbital prefrontal cortex dopamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology 175:206–214
Kheramin S, Body S, Miranda Herrera F, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM (2005) The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: implications for models of inter-temporal choice. Behav Brain Res 156:145–152
Killeen PR (2005) Gradus ad Parnassum: Ascending strength gradients or descending memory traces? Behav Brain Sci 28:432–434
Killeen PR, Fetterman JG (1988) A behavioral theory of timing. Psychol Rev 95:274–295
Killeen PR, Fetterman JG, Bizo LA (1997) Time’s causes. In: Bradshaw CM, Szabadi E (eds) Time and behaviour: psychological and neurobehavioural analyses. Elsevier, Amsterdam
Lindstrom MJ, Bates DM (1990) Nonlinear mixed-effects models for repeated measures data. Biometrics 46:673–687
Logue AW (1988) Research on self-control: an integrating framework. Behav Brain Sci 11:665–678
Mazur JE (1987) An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H (eds) Quantitative analyses of behavior: V. The effect of delay and of intervening events on reinforcement value. Lawrence Erlbaum, Hillsdale, New Jersey, pp 55–73
Mazur JE (2006) Mathematical models and the experimental analysis of behavior. J Exp Anal Behav 85:285–291
Mobini S, Chiang TJ, Al-Ruwaitea AS, Ho MY, Bradshaw CM, Szabadi E (2000a) Effect of central 5-hydroxytryptamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology 149:313–318
Mobini S, Body S, Ho M-Y, Bradshaw CM, Szabadi E (2000b) Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology 160:290–298
Monterosso J, Ainslie G (1999) Beyond discounting: possible experimental models of impulse control. Psychopharmacology 146:339–347
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic, San Diego
Pinheiro JC, Bates DM (2004) Mixed-effects models in S and S-plus. Springer, New York
Pothuizen HHJ, Jongen-Relo AL, Feldon J, Yee BK (2005) Double dissociation of the effects of selective nucleus accumbens core and shell lesions on impulsive-choice behaviour and salience learning in rats. Eur J Neurosci 22:2605–2616
Rachlin H (1974) Self-control. Behaviorism 2:94–107
Rachlin H (2006) Notes on discounting. J Exp Anal Behav 85:425–435
Richards JB, Sabol KE, de Wit H (1999) Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology 146:432–439
Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth FS (2006) Separate neural pathways process different decision costs. Nat Neurosci 9:1161–1168
Schwarcz R, Whetsell WO, Mangano RM (1983) Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat-brain. Science 219:316–318
Snedecor GW, Cochran WG (1989) Statistical methods, 8th edn. Iowa State University Press
Wade TR, de Wit H, Richards JB (2000) Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology 150:90–101
Winstanley CA, Theobald DEH, Dalley JW, Robbins TW (2003) Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice in rats. Psychopharmacology 170:320–331
Winstanley CA, Theobald DEH, Cardinal RN, Robbins TW (2004) Contrasting roles for basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci 24:4718–4722
Winstanley CA, Baunez C, Theobald DEH, Robbins TW (2005) Lesions to the subthalamic nucleus decrease impulsive choice but impair autoshaping in rats: the importance of the basal ganglia in Pavlovian conditioning and impulse control. Eur J Neurosci 21:3107–3116
Wogar MA, Bradshaw CM, Szabadi E (1993) Effects of lesions of the ascending 5-hydroxytryptaminergic pathways on choice between delayed reinforcers. Psychopharmacology 111:239–243
Zar JH (1999) Biostatistical analysis, fourth edition. Prentice-Hall, Upper Saddle River, NJ
Acknowledgements
This work was supported by the Wellcome Trust. We are grateful to Ms. V.K. Bak and Mr R.W. Langley for skilled technical help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bezzina, G., Cheung, T.H.C., Asgari, K. et al. Effects of quinolinic acid-induced lesions of the nucleus accumbens core on inter-temporal choice: a quantitative analysis. Psychopharmacology 195, 71–84 (2007). https://doi.org/10.1007/s00213-007-0882-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0882-0