Abstract
Rationale
Episodic social defeat stress results in cross-sensitization to cocaine, characterized by augmentation of locomotor activity, dopamine (DA) levels in the nucleus accumbens (NAc), and cocaine self-administration during a 24-h “binge” in male rats. However, females are more vulnerable than males at each phase of cocaine addiction, and while these sex differences have been replicated in rats, the role of social stress in females remains largely neglected.
Objective
This study examined sex and estrous cycle differences in behavioral and dopaminergic cross-sensitization to cocaine, as well as cocaine taking in an unlimited-access self-administration “binge.”
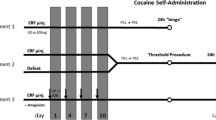
Methods
Long-Evans rats underwent episodic social defeat and were assessed 10 days later for either (1) behavioral sensitization, as determined by locomotor activity in response to acute cocaine (10 mg/kg, i.p.), (2) neural sensitization, as determined by in vivo microdialysis of DA in the NAc shell in response to acute cocaine, or (3) intravenous self-administration of cocaine (0.3 mg/kg/infusion) in an unlimited-access “binge.”
Results
Social defeat stress resulted in behavioral and dopaminergic cross-sensitization in both sexes, but the effect was larger and longer lasting in stressed females. Furthermore, while stress engendered a longer “binge” in both sexes, females had a significantly longer “binge” duration than males.
Conclusions
These data suggest that socially stressed females exhibit a larger and longer lasting behavioral and neural cross-sensitization, as well as more dysregulated cocaine taking, than males possibly due to different alterations in the dopaminergic response in the NAc. Furthermore, estrogens appear to play a facilitatory role in both behavioral and dopaminergic sensitization.





Similar content being viewed by others
References
Anker JJ, Carroll ME (2011) Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci 8:73–96
Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H (2005) Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 180:169–176
Becker JB, Hu M (2008) Sex differences in drug abuse. Front Neuroendocrinol 29:36–47
Becker JB, Molenda H, Hummer DL (2001) Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci 937:172–187
Boyson CO, Miguel TT, Quadros IM, Debold JF, Miczek KA (2011) Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl) 218:257–269
Cabib S, Puglisi-Allegra S (2012) The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev 36:79–89
Carroll ME, Anderson MM, Morgan AD (2007) Higher locomotor response to cocaine in female (vs. male) rats selectively bred for high (HiS) and low (LoS) saccharin intake. Pharmacol Biochem Behav 88:94–104
Castner SA, Xiao L, Becker JB (1993) Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res 610:127–134
Chen K, Kandel D (2002) Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol Depend 68:65–85
Covington HE 3rd, Miczek KA (2001) Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 158:388–398
Covington HE 3rd, Miczek KA (2005) Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 183:331–340
Covington HE 3rd, Kikusui T, Goodhue J, Nikulina EM, Hammer RP Jr, Miczek KA (2005) Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology 30:310–321
Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB (2011) Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ 2:3
De Wit H, Wise RA (1977) Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can J Psychol 31:195–203
Fox HC, Sinha R (2009) Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harv Rev Psychiatry 17:103–119
Fox HC, Hong KI, Siedlarz K, Sinha R (2008) Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology 33:796–805
Haney M, Maccari S, Le Moal M, Simon H, Piazza PV (1995) Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res 698:46–52
Hu M, Becker JB (2003) Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci 23:693–699
Ignjatova L, Raleva M (2009) Gender difference in the treatment outcome of patients served in the mixed-gender program. Bratisl Lek Listy 110:285–289
Kalivas PW, Duffy P (1993) Time course of extracellular dopamine and behavioral sensitization to cocaine. II. Dopamine perikarya. J Neurosci 13:276–284
Lynch WJ (2008) Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 197:237–246
Lynch WJ, Carroll ME (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 144:77–82
Lynch WJ, Taylor JR (2004) Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology 29:943–951
McCance-Katz EF, Carroll KM, Rounsaville BJ (1999) Gender differences in treatment-seeking cocaine abusers—implications for treatment and prognosis. Am J Addict 8:300–311
McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI (1996) Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis 184:616–622
Miczek KA (1979) A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 60:253–259
Miczek KA, Mutschler NH, van Erp AM, Blank AD, McInerney SC (1999) d-Amphetamine “cue” generalizes to social defeat stress: behavioral sensitization and attenuated accumbens dopamine. Psychopharmacology (Berl) 147:190–199
Miczek KA, Nikulina EM, Shimamoto A, Covington HE 3rd (2011) Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci 31:9848–9857
National Research Council (2011) Guide for the care and use of laboratory animals: eighth edition. The National Academies Press
Nikulina EM, Covington HE 3rd, Ganschow L, Hammer RP Jr, Miczek KA (2004) Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience 123:857–865
O'Brien MS, Anthony JC (2005) Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology 30:1006–1018
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates, 3rd edn. Academic, San Diego
Puig-Ramos A, Santiago GS, Segarra AC (2008) U-69593, a kappa opioid receptor agonist, decreases cocaine-induced behavioral sensitization in female rats. Behav Neurosci 122:151–160
Roberts DC, Bennett SA, Vickers GJ (1989) The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 98:408–411
Roth ME, Carroll ME (2004) Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav 78:199–207
SAMHSA (2010) Results from the 2010 National Survey on Drug Use and Health: National Findings. In: Studies OoA (ed) (NSDUH Series H-36). SAMHSA, Rockville, MD
Sell SL, Scalzitti JM, Thomas ML, Cunningham KA (2000) Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther 293:879–886
Sell SL, Thomas ML, Cunningham KA (2002) Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend 67:281–290
Shaham Y, Erb S, Stewart J (2000) Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev 33:13–33
Shimamoto A, Debold JF, Holly EN, Miczek KA (2011) Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology (Berl) 218:271–279
Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158:343–359
Sinha R (2008) Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141:105–130
Sinha R (2009) Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol 14:84–98
Sinha R, Li CS (2007) Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev 26:25–31
Sircar R, Kim D (1999) Female gonadal hormones differentially modulate cocaine-induced behavioral sensitization in Fischer, Lewis, and Sprague-Dawley rats. J Pharmacol Exp Ther 289:54–65
Staples RE, Geils HD (1965) An observation on the vaginal smear of the rat. J Endocrinol 32:263–264
Tidey JW, Miczek KA (1996) Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res 721:140–149
Tidey JW, Miczek KA (1997) Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 130:203–212
Tornatzky W, Miczek KA (1993) Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav 53:983–993
van Haaren F, Meyer ME (1991) Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav 39:923–927
Xiao L, Becker JB (1994) Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett 180:155–158
Zhao W, Becker JB (2010) Sensitization enhances acquisition of cocaine self-administration in female rats: estradiol further enhances cocaine intake after acquisition. Horm Behav 58:8–12
Acknowledgments
This research was supported by NIH Grant DA031734. We would like to thank Rachel Doyle, Melanie Monroe, and Andrew Terrano for their great help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holly, E.N., Shimamoto, A., DeBold, J.F. et al. Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology 224, 179–188 (2012). https://doi.org/10.1007/s00213-012-2846-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2846-2