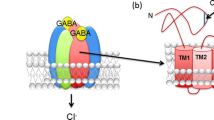
Abstract
Rationale
Neurosteroids are steroids synthesized within the brain with rapid effects on neuronal excitability. Allopregnanolone, allotetrahydrodeoxycorticosterone, and androstanediol are three widely explored prototype endogenous neurosteroids. They have very different targets and functions compared to conventional steroid hormones. Neuronal γ-aminobutyric acid (GABA) type A (GABAA) receptors are one of the prime molecular targets of neurosteroids.
Objective
This review provides a critical appraisal of recent advances in the pharmacology of endogenous neurosteroids that interact with GABAA receptors in the brain. Neurosteroids possess distinct, characteristic effects on the membrane potential and current conductance of the neuron, mainly via potentiation of GABAA receptors at low concentrations and direct activation of receptor chloride channel at higher concentrations. The GABAA receptor mediates two types of inhibition, now characterized as synaptic (phasic) and extrasynaptic (tonic) inhibition. Synaptic release of GABA results in the activation of low-affinity γ2-containing synaptic receptors, while high-affinity δ-containing extrasynaptic receptors are persistently activated by the ambient GABA present in the extracellular fluid. Neurosteroids are potent positive allosteric modulators of synaptic and extrasynaptic GABAA receptors and therefore enhance both phasic and tonic inhibition. Tonic inhibition is specifically more sensitive to neurosteroids. The resulting tonic conductance generates a form of shunting inhibition that controls neuronal network excitability, seizure susceptibility, and behavior.
Conclusion
The growing understanding of the mechanisms of neurosteroid regulation of the structure and function of the synaptic and extrasynaptic GABAA receptors provides many opportunities to create improved therapies for sleep, anxiety, stress, epilepsy, and other neuropsychiatric conditions.









Similar content being viewed by others
Abbreviations
- 3α-HSOR:
-
3α-Hydroxysteroid oxidoreductase
- 17PA:
-
(3α,5α)-17-Phenylandrost-16-en-3-ol
- AP:
-
Allopregnanolone (3α-hydroxy-5α-pregnan-20-one)
- CGC:
-
Cerebellar granule cell
- CNS:
-
Central nervous system
- DGGC:
-
Dentate gyrus granule cell
- DHEAS:
-
Dehydroepiandrosterone sulfate
- GABARAP:
-
GABAA receptor-associated protein
- IPSC:
-
Inhibitory postsynaptic current
- NMDA:
-
N-Methyl-d-aspartate
- P:
-
Progesterone
- PS:
-
Pregnenolone sulfate
- TBI:
-
Traumatic brain injury
- TBPS:
-
tert-Butylbicyclophosphorothionate
- THDOC:
-
Allotetrahydrodeoxycorticosterone (3α,21-dihydroxy-5α-pregnan-20-one)
- THIP:
-
4,5,6,7-Tetrahydroisoxazolo[5,4-c]pyridine-3-ol
- TLE:
-
Temporal lobe epilepsy
- TSPO:
-
Translocator protein
References
Abramian AM, Comenencia-Ortiz E, Vithlani M, Tretter EV, Sieghart W, Davies PA, Moss SJ (2010) Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J Biol Chem 285:41795–41805
Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A (2006) Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci U S A 103:14602–14607
Akk G, Bracamontes J, Steinbach JH (2001) Pregnenolone sulfate block of GABAA receptors: mechanism and involvement of a residue in the M2 region of the α-subunit. J Physiol 532:673–684
Akk G, Covery DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S (2009) The influence of the membrane on neurosteroid actions at GABAA receptors. Psychoneuroendocrinology 34S:S59–S66
Akk G, Shu H, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S (2005) Neurosteroid access to the GABAA receptor. J Neurosci 25:11605–11613
Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR (2006) L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for α5-containing GABAA receptors. Neuropharmacology 51:1023–1029
Attwell D, Barbour B, Szatkowski M (1993) Nonvesicular release of neurotransmitter. Neuron 11:401–407
Bai D, Zhu G, Pennefather P, Jackson MF, Macdonald JF, Orser BA (2001) Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol 59:814–824
Baker C, Sturt BL, Bamber BA (2010) Multiple roles for the first transmembrane domain of GABAA receptor subunits in neurosteroid modulation and spontaneous channel activity. Neurosci Lett 473:242–247
Barha CK, Ishrat T, Epp JR, Galea LA, Stein DG (2011) Progesterone treatment normalizes the levels of cell proliferation and cell death in the dentate gyrus of the hippocampus after traumatic brain injury. Exp Neurobiol 231:72–81
Barmashenko G, Hefft S, Aertsen A, Kirschstein T, Kohling R (2011) Positive shifts of the GABAA receptor reversal potential due to altered chloride homeostasis is widespread after status epilepticus. Epilepsia 52:1570–1578
Bateson AN (2002) Basic pharmacological mechanisms involved in benzodiazepine tolerance and withdrawal. Curr Pharm Des 8:5–21
Baulieu EE (1981) Steroid hormones in the brain: several mechanisms? In: Fuxe F, Gustafsson JA, Wetterberg L (eds) Steroid hormone regulation of the brain. Pergamon, Oxford, pp 3–14
Baumann SW, Baur R, Sigel E (2002) Forced subunit assembly in α1β2γ2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem 277:46020–46025
Baumann SW, Baur R, Sigel E (2003) Individual properties of the two functional agonist sites in GABAA receptors. J Neurosci 23:11158–11166
Benson JA, Löw K, Keist R, Mohler H, Rudolph U (1998) Pharmacology of recombinant gamma-aminobutyric acidA receptors rendered diazepam-insensitive by point-mutated alpha-subunits. FEBS Lett 431:400–404
Belelli D, Bolger MB, Gee KW (1989) Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Eur J Pharmacol 166:325–329
Belelli D, Casula A, Ling A, Lambert JJ (2002) The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology 43:651–661
Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW (2009) Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci 29:12757–12763
Belelli D, Lambert JL (2005) Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci 6:565–575
Benke D, Michel C, Mohler H (1997) GABAA receptors containing the α4 subunit: prevalence, distribution, pharmacology, and subunit architecture in situ. J Neurochem 69:806–814
Bianchi MT, Haas KF, Macdonald RL (2001) Structural determinants of fast desensitization and desensitization–deactivation coupling in GABAA receptors. J Neurosci 21:1127–1136
Bianchi MT, Haas KF, Macdonald RL (2002) α1 and α6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABAA receptors containing the δ subunit. Neuropharmacology 43:492–502
Bianchi MT, Macdonald RL (2003) Neurosteroids shift partial agonist activation of GABAA receptor channels from low- to high-efficacy gating patterns. J Neurosci 23:10934–10943
Biggio G, Follesa P, Sanna E, Purdy RH, Concas A (2001) GABAA-receptor plasticity during long-term exposure to and withdrawal from progesterone. Int Rev Neurobiol 46:207–241
Biggio F, Gorini G, Caria S, Murru L, Mostallino MC, Sanna E, Follesa P (2006) Plastic neuronal changes in GABAA receptor gene expression induced by progesterone metabolites—in vitro molecular and functional studies. Pharm Biochem Behav 84:545–554
Boehm SL 2nd, Homanics GE, Blednov YA, Harris RA (2006) δ-Subunit containing GABAA receptor knockout mice are less sensitive to the actions of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridine-3-ol. Eur J Pharmacol 541:158–162
Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ (2006) Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J 25:4381–4389
Bonin RP, Martin LJ, MacDonald JF, Orser BA (2007) α5GABAA receptors regulate the intrinsic excitability of hippocampal pyramidal neurons. J Neurophysiol 98:2244–2254
Bosman LW, Rosahl TW, Brussard AB (2002) Neonatal development of the rat visual cortex: synaptic function of GABAA receptor α subunits. J Physiol 545(Pt 1):169–181
Bracamontes J, McCollum M, Esch C, Li P, Ann J, Steinbach JH, Akk G (2011) Occupation of either site for the neurosteroid allopregnanolone potentiates the opening of the GABAA receptor induced from either transmitter binding site. Mol Pharmacol 80:79–86
Bright DP, Renzi M, Bartram J, McGee TP, MacKinzie G, Hosie AM, Farrant M, Brickley SG (2011) Profound desensitization by ambient GABA limits activation of δ-containing GABAA receptors during spillover. J Neurosci 31:753–763
Briyal S, Reddy DS (2007) Antiepileptic efficacy of the delta-subunit-preferring GABAA receptor agonist gaboxadol on spontaneous recurrent seizures in a rat model of temporal lobe epilepsy. Soc Neurosci Abst 24(375.22):36
Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Kelly ME, Coulter DA (2001) γ-Aminobutyric acidA receptor subunit expression predicts functional changes in hippocampal dentate granule cells during postnatal development. J Neurochem 77:1266–1278
Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA (2002) Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol 136:965–974
Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicnell RJ, Herbison AE (1997) Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABAA receptor subunit expression. Neuron 19:1103–1114
Brussaard AB, Koksma JJ (2003) Conditional regulation of neurosteroid sensitivity of GABAA receptors. Ann N Y Acad Sci 1007:29–36
Brussaard AB, Herbison AE (2000) Long-term plasticity of postsynaptic GABAA receptor function in the adult brain: insights from oxytocin neurone. Trends Neurosci 23:190–195
Buhr A, Wagner FK, Sieghart W, Sigel E (2001) Two novel residues in M2 of the γ-aminobutyric acid type A receptor affecting gating by GABA and picrotoxin affinity. J Biol Chem 276:7775–7781
Calza A, Sogliano C, Santoru F, Marra C, Angioni MM, Mostallino MC, Biggio G, Concas A (2010) Neonatal exposure to estradiol in rats influences neuroactive steroid concentrations, GABAA receptor expression, and behavioral sensitivity to anxiolytic drugs. J Neurochem 113:1285–1295
Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newll JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA et al (2004) Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A 101:3662–3667
Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW (1997) Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor. J Pharmacol Exp Ther 280:1284–1295
Cavelier P, Hamann M, Rossi D, Mobbs P, Attwell D (2005) Tonic excitation and inhibition of neurons: ambient transmitter sources and computational consequences. Prog Biohphys Mol Biol 87:3–16
Chandra D, Halonen LM, Linden AM, Procaccini C, Hellsten K, Homanics GE, Korpi ER (2010) Prototypic GABAA receptor agonist muscimol acts preferentially through forebrain high-affinity binding sites. Neuropsychopharmacology 35:999–1007
Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL et al (2006) GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci U S A 103:15230–15235
Chen ZW, Manion B, Townsend RR, Reichert DE, Covey DF, Steinbach JH, Sieghart W, Fuchs K, Evers AS (2012) Neurosteroid analog photolabeling of a site in the third transmembrane domain of the β3 subunit of the GABAA receptor. Mol Pharmacol 82:408–419
Cherubini E, Gaiarsa JL, Ben-Ari Y (1991) GABA: an excitatory transmitter in early postnatal life. Trends Neurosci 14:515–519
Chisari M, Eisenman LN, Krishnan K, Bandyopadhyaya AK, Wang C, Taylor A, Benz A, Covey DF, Zorumski CF, Mennerick S (2009) The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low0affinity interaction. J Neurophysiol 102:1254–1264
Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF (2010) The sticky issue of neurosteroids and GABA-A receptors. Trends Neurosci 33:299–306
Chiu CS, Brickley S, Jensen K, Southwell A, Mckinney S, Cull-Candy S, Mody I, Lester HA (2005) GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J Neurosci 25:3234–3245
Citraro R, Russo E, Di Paola ED, Ibbadu GF, Gratteri S, Marra R, De Sarro G (2006) Effects of some neurosteroids injected into some brain areas of WAG/Rij rats, an animal model of generalized absence epilepsy. Neuropharmacology 50:1059–1071
Clarke RS, Dundee JW, Carson IW (1973) Proceedings: a new steroid anaesthetic-althesin. Proc R Soc Med 66:1027–1030
Cohen AS, Lin DD, Quirk GL, Coulter DA (2003) Dentate granule cell GABAA receptors in epileptic hippocampus: enhanced synaptic efficacy and altered pharmacology. Eur J Neurosci 17:1607–1616
Compagnone NA, Mellon SH (2000) Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol 21:1–56
Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G (1998) Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U S A 95:13284–13289
Coulter DA, Carlson GC (2007) Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog Brain Res 163:235–243
Covey DF, Nathan D, Kalkbrenner M, Nilsson KR, Hu Y, Zorumsk CF, Evers AS (2000) Enantioselectivity of pregnanolone-induced γ-aminobutyric acidA receptor modulation and anesthesia. J Pharm Exp Ther 293:1009–1116
Crawley JN, Glowa JR, Majewska MD, Paul SM (1986) Anxiolytic activity of endogenous adrenal steroid. Brain Res 398:382–385
Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejin L (2002) Paracrine intracellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron 36:1051–1061
Derry JMC, Dunn SMJ, Davies M (2004) Identification of a residue in the γ-aminobutyric acid type A receptor α subunit that differentially affects diazepam-sensitive and -insensitive benzodiazepine site binding. J Neurochem 88:1431–1438
Devaud LL, Purdy RH, Finn DA, Morrow AL (1996) Sensitization of γ-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharm Exp Ther 278:510–517
Diaz MR, Wadleigh A, Hughes BA, Woodward JJ, Valenzuela CF (2012) Bestrophin1 channels are insensitive to ethanol and do not mediate tonic GABAergic currents in cerebellar granule cells. Front Neurosci 5:148
Dibbens LM, Feng HJ, Richards MC, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE et al (2004) GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet 13:1315–1319
Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H (2009) Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol 30:259–301
Duveau V, Laustela S, Barth L, Gianolini F, Vogt KE, Keist R, Chandra D, Homanics GE, Rudolph U, Fritschy JM (2011) Spatiotemporal specificity of GABAA receptor-mediated regulation of adult hippocampal neurogenesis. Eur J Neurosci 34:362–373
Ebert B, Thompson SA, Saounatsou K, McKernan R, Krogsgaard-Larsen P, Wafford KA (1997) Differences in agonist/antagonist binding affinity and receptor transduction using recombinant human γ-aminobutyric acid type A receptors. Mol Pharmacol 52:1150–1156
Ernst M, Brauchart D, Boresch S, Sieghart W (2003) Comparative modeling of GABAA receptors: limits, insights, future developments. Neuroscience 119:933–943
Farrant M, Nusser Z (2005) Variations on an inhibitory them: phasic and tonic activation of GABAA receptors. Nat Rev 6:215–229
Feng HJ, Kang JQ, Song L, Dibbens L, Mulley J, Macdonald RL (2006) δ subunit susceptibility variants E177A R220H associated with complex epilepsy alter channel gating and surface expression of α4β2δ GABAA receptors. J Neurosci 26:1499–1506
Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS (2011) GABA transport modulates the ethanol sensitivity of tonic inhibition in the rat dentate gyrus. Alcohol 45:577–583
Freiss E, Schiffelholz T, Steckler T, Steiger A (2000) Dehydroepiandorsterone—a neurosteroid. Eur J Clin Invest Suppl 3:46–50
Frye CA (1995) The neuroactive steroid 3α,5α-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res 696:113–120
Galanopoulou AS (2008a) Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABAA receptors. J Neurosci 28:1557–1567
Galanopoulou AS (2008b) GABAA receptors in normal development and seizures: friends or foes. Curr Neuropharmacol 6:1–20
Gangisetty O, Reddy DS (2009) The optimization of TaqMan real-time RT-PCR assay for transcriptional profiling of GABAA receptor subunit plasticity. J Neurosci Methods 181:58–66
Gangisetty O, Reddy DS (2010) Neurosteroid withdrawal regulates GABAA receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience 170:865–880
Gartside SE, Griffith NC, Kaura V, Ingram CD (2010) The neurosteroid dehydroepiandrosterone (DHEA) and its metabolites alter 5-HT neuronal activity via modulation of GABAA receptors. J Psychopharmacol 24:1717–1724
Gee KW, Bolger MB, Brinton RE, Coirini H, McEwen BS (1988) Steroid modulation of the chloride ionophore in rat brain: structure–activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther 246:803–812
Gibbs JW 3rd, Shumate MD, Coulter DA (1997) Differential epilepsy-associated alterations in postsynaptic GABAA receptor function in dentate granule and CA1 neurons. J Physiol 77:1924–1938
Gibson CJ, Meyer RC, Hamm RJ (2010) Traumatic brain injury and the effects of diazepam, diltiazem, and MK-801 on GABA-A receptor subunit expression in rat hippocampus. J Biomed Sci 17:38
Glykys J, Mann EO, Mody I (2008) Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci 28:1421–1426
Glykys J, Mody I (2007) Activation of GABAA receptors: views from outside the synaptic cleft. Neuron 56:763–770
Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I (2007) A new naturally occurring GABAA receptor subunit with high sensitivity to ethanol. Nat Neurosci 10:40–48
Goodkin HP, Yeh JL, Kapur J (2005) Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci 25:5511–5520
Gulinello M, Smith SS (2003) Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharm Exp Ther 305:541–548
Hadley SH, Amin J (2007) Rat α6β2δ GABAA receptors exhibit two distinct and separable agonist affinities. J Physiol 581:1001–1018
Hajos N, Nusser Z, Rancz EA, Freund TF, Mody I (2000) Cell type- and synapse-specific variability in synaptic GABAA receptor occupancy. Eur J Neurosci 12:810–818
Hamann M, Rossi DJ, Attwell D (2002) Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron 33:625–633
Han JW, Nakamura M, Choi IS, Cho JH, Park HM, Lee MG, Choi BJ, Jang HJ, Jang IS (2009) Differential pharmacological properties of GABAA receptors in axon terminals and soma of dentate gyrus granule cells. J Neurochem 109:995–1007
Hancar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW (2006) Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to α4/6β3δ GABAA receptors. Proc Natl Acad Sci U S A 103:8546–8551
Harney SC, Frenquelli BG, Lambert JJ (2003) Phosphorylation influences neurosteroid modulation of synaptic GABAA receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology 45:873–883
Harrison NL, Simmonds MA (1984) Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res 323:287–292
Harrison NL, Vicini S, Barker JL (1987) A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurosci 7:604–609
He J, Hoffman SW, Stein DG (2004) Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci 22:19–31
Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL (1992) The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res 7(Suppl):273–280
Herd MB, Belelli LJJ (2007) Neurosteroid modulation of synaptic and extrasynaptic GABAA receptors. Pharamcol Ther 116:20–34
Herd MB, Haythornwaite AR, Rosahl TW, Wafford KA, Homanics GE, Lambert JJ, Belelli D (2008) The expression of GABAA β subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J Physiol 586:989–1004
Herzog AG (1999) Psychoneuroendocrine aspects of temporolimbic epilepsy: part II: epilepsy and reproductive steroids. Psychosomatics 40:102–108
Herzog AG (2009) Hormonal therapies: progesterone. Neurotherapeutics 6:383–391
Holter NI, Zylla MM, Zuber N, Bruehl C, Draguhn A (2010) Tonic GABAergic control of mouse dentate granule cells during postnatal development. Eur J Neurosci 32:1300–1309
Horak M, Vlcek K, Petrovic M, Chodounska H, Vyklicky L Jr (2004) Molecular mechanism of pregnenolone sulfate action at NR1/NR2B receptors. J Neurosci 24:10318–10325
Hosie AM, Clarke L, da Silva H, Smart TG (2009) Conserved site for neurosteroid modulation of GABAA receptors. Neuropharmacology 56:149–154
Hosie AM, Dunne EL, Harvey RJ, Smart TG (2003) Zinc-mediated inhibition of GABAA receptors: discrete binding sites underlie subtype specificity. Nat Neurosci 6:362–369
Hosie AM, Wilkins ME, da Silva HMA, Smart TG (2006) Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nat Lett 444:486–489
Hosie AM, Wilkins ME, Smart TG (2007) Neurosteroid binding sites on GABAA receptors. Pharmacol Ther 116:7–19
Houser CR, Esclapez M (2003) Downregulation of the α5 subunit of the GABAA receptor in the pilocarpine model of temporal lobe epilepsy. Hippocampus 13:633–645
Houston CM, McGee TP, Mackenzie G, Troyano-Cuturi K, Rodriguez PM, Kutsarova E, Diamanti E, Hosie AM, Franks NP, Brickley SG (2012) Are extrasynaptic GABAA receptors important targets for sedative/hypnotic drugs? J Neurosci 32:3887–3897
Hsu FC, Smith SS (2003) Progesterone withdrawal reduces paired-pulse inhibition in rat hippocampus: dependence on GABAA receptor α4 subunit upregulation. J Neurophysiol 89:186–198
Hsu FC, Waldeck R, Faber DS, Smith SS (2003) Neurosteroid effects on GABAergic synaptic plasticity in hippocampus. J Neurophysiol 89:1929–1940
Ing T, Poulter MO (2007) Diversity of GABAA receptor synaptic currents on individual pyramidal cortical neurons. Eur J Neurosci 25:723–734
Iwakiri M, Mizukami K, Ishikawa M, Asada T (2006) GABAA receptor γ subunits in the hippocampus of the rat after perforant pathway lesion. Neurosci Lett 394:88–91
Jacob TC, Moss SJ, Jurd R (2008) GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci 9:331–343
Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL (2008) Taurine is a potent activator of extrasynaptic GABAA receptors in the thalamus. J Neurosci 28:106–115
Jefcoate C (2002) High-flux mitochondrial cholesterol trafficking, a specialized function of the adrenal cortex. J Clin Invest 110:881–890
Jursky F, Fuchs K, Buhr A, Tretter V, Sigel E, Sieghart W (2000) Identification of amino acid residues of GABAA receptor subunits contributing to the formation and activity of the tert-butylbicyclophosphorothionate binding site. J Neurochem 74:1310–1316
Kaminski RM, Fu Z, Venkatesan K, Mazzuferi M, Leclerq K, Seutin V, Vicini S (2011) 11-Deoxycortisol impedes GABAergic neurotransmission and induces drug-resistant status epilepticus in mice. Neuropharmacology 60:1098–1108
Kaminski RM, Marini H, Kim WJ, Rogawski MA (2005) Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia 46:819–827
Kaminski RM, Marini H, Ortinski PI, Vicini S, Rogawski MA (2006) The pheromone androstenol (5α-androst-16-en-3α-ol) is a neurosteroid positive modulator of GABAA receptors. J Pharmacol Exp Ther 317:694–703
Katona BW, Krishnan K, Cai ZY, Manion BD, Benz A, Taylor A, Evers AS, Zorumski CF, Mennerick S, Covey DF (2008) Neurosteroid analogues. 12. Potent enhancement of GABA-mediated chloride currents GABAA receptors by ent-androgens. Eur J Med Chem 43:107–113
Kaur KH, Baur R, Sigel E (2009) Unanticipated structural and functional properties of δ-subunit-containing GABAA receptors. J Biol Chem 284:7889–7896
Kelley MH, Kuroiwa M, Taguchi N, Herson PS (2011) Sex difference in sensitivity to allopregnanolone neuroprotection in mice correlates with effect on spontaneous inhibitory post synaptic currents. Neuropharmacology 61:724–729
Kelley SP, Alan JK, O’Buckley TK, Mennerick S, Krishnan K, Covey DF, Morrow AL (2007) Antagonism of neurosteroid modulation of native gamma-aminobutyric acid receptors by (3α,5α)-17-phenylandrost-16-en-3-ol. Eur J Pharmacol 572:94–101
Khanna M, Qin KN, Cheng KC (1995) Distribution of 3α-hydroxysteroid dehydrogenase in rat brain and molecular cloning of multiple cDNAs encoding structurally related proteins in humans. J Steroid Biochem Mol Biol 53:41–46
Kharlamov EA, Lepsveridze E, Meparishvili M, Solomonia RO, Lu B, Miller ER, Kelly KM, Mtchedlishvili Z (2011) Alterations of GABAA and glutamate receptor subunits and heat shock protein in rat hippocampus following traumatic brain injury and in posttraumatic epilepsy. Epilepsy Res 95:20–34
Kia A, Ribiero F, Nelson R, Gavriolovici C, Ferguson SSG, Poulter MO (2011) Kindling alters neurosteroid-induced modulation of phasic and tonic GABAA receptor-mediated currents: role of phosphorylation. J Neurochem 116:1043–1056
Kim BG, Cho JH, Choi IS, Lee MG, Jang IS (2011) Modulation of presynaptic GABAA receptors by endogenous neurosteroids. Br J Pharmacol 164:1698–1710
Kita A, Furukawa K (2008) Involvement of neurosteroids in the anxiolytic-like effects of AC-5216 in mice. Pharmacol Biochem Behav 89:171–178
Knoflach F, Dietmar B, Wang Y, Scheurer L, Luddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA (1996) Pharmacological modulation of the diazepam-insensitive recombinant γ-aminobutyric acidA receptors α4β2γ2 and α6β2γ2. Mol Pharmacol 50:1253–1261
Kokate TG, Cohen AL, Karp E, Rogawski MA (1996) Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology 35:1049–1056
Kokate TG, Juhng KN, Kirkby RD, Llamas J, Yamaguchi S, Rogawski MA (1999) Convulsant actions of the neuroactive steroid pregnenolone sulfate in mice. Brain Res 831:119–124
Kokate TG, Svensson BE, Rogawski MA (1994) Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther 270:1223–1229
Kokate TG, Yamaguchi S, Pannell LK, Rajamani U, Carroll DM, Grossman AB, Rogawski MA (1998) Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. J Pharmacol Exp Ther 287:553–558
Korneyev A, Pan BS, Polo A, Romeo E, Guidotti A, Costa E (1993) Stimulation of brain pregnenolone synthesis by mitochondrial diazepam binding inhibitor receptor ligands in vivo. J Neurochem 61:1515–1524
Korpi ER, Kuner T, Seeburg PH, Lüddens H (1995) Selective antagonist for the cerebellar granule cell-specific gamma-aminobutyric acid type A receptor. Mol Pharmacol 47:283–289
Korpi ER, Lüddens H (1997) Furosemide interaction with brain GABAA receptors. Br J Pharmacol 120:741–748
Korpi ER, Mihalek RM, Sinkkonen ST, Hauer B, Hevers W, Homanics GE, Sieghart W, Lüddens H (2002) Altered receptor subtypes in the forebrain of GABAA receptor δ subunit-deficient mice: recruitment of γ2 subunits. Neuroscience 109:733–743
Kralic JE, Sidler C, Parpan F, Homanics GE, Morrow AL, Fritschy JM (2006) Compensatory alteration of inhibitory synaptic current in cerebellum and thalamus of γ-aminobutyric acid type A receptor α1 subunit knockout mice. J Comp Neurol 495:408–421
Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Lol CJ, Stromgaard K, Gether U (2011) SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev 63:585–640
Kulkarni SK, Reddy DS (1995) Neuroactive steroids: a new class of neuromodulators. Drugs Today 31:433–455
Kuver A, Shen H, Smith SS (2012) Regulation of the surface expression of α4β2δ GABAA receptors by high efficacy states. Brain Res 1463:1–20
Lagrange AH, Botzolakis EJ, Macdonald RL (2007) Enhanced macroscopic desensitization shapes the response of α4 subtype-containing GABAA receptors to synaptic and extrasynaptic GABA. J Physiol 578:655–676
Lambert JJ, Belelli D, Hill-Venning C, Peters JA (1995) Neuroactive steroids and GABAA receptor function. Trends Pharmacol Sci 16:295–303
Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA (2003) Neurosteroid modulation of GABAA receptors. Prog Neurobiol 71:67–80
Lambert JJ, Cooper MA, Simmons RDJ, Weir CJ, Belelli D (2009) Neurosteroids: endogenous allosteric modulators of GABAA receptors. Psychoneuroendocrinology 34S:S48–S58
Lan NC, Gee KW, Bolger MB, Chen JS (1991) Differential responses of expressed recombinant human γ-aminobutyric acidA receptors to neurosteroids. J Neurochem 57:1818–1821
Laurie DJ, Wisden W, Seeburg PH (1992) The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci 12:4151–4172
Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE (1997) Activation and deactivation rates of recombinant GABAA receptor channels are dependent on α-subunit isoform. Biophys J 73:2518–2526
Lawrence C, Martin BS, Sun C, Williamson J, Kapur J (2010) Endogenous neurosteroid synthesis modulates seizure frequency. Ann Neurol 67:689–693
Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ (2010) Channel-mediated tonic GABA release from glia. Science 330:790–796
Leil TA, Chen ZW, Chang CS, Olsen RW (2004) GABAA receptor-associated protein traffics GABAA receptors to the plasma membrane in neurons. J Neurosci 24:11429–11438
Lerma J, Herranz AS, Herreras O, Abraira V, Martín del Río R (1986) In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res 384:145–155
Li GD, Chiara DC, Cohen JB, Olsen RW (2009) Neurosteroids allosterically modulate binding of the anesthetic etomidate to γ-aminobutyric acid type A receptors. J Biol Chem 284:11771–11775
Liang J, Suryanarayanan A, Chandra D, Homanics GE, Olsen RW, Spigelman I (2008) Functional consequences of GABAA receptor α4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol Clin Exp Res 32:19–26
Lothman EW, Stringer JL, Bertram EH (1992) The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res Suppl 7:301–313
Luscher B, Fuchs T, Kilpatrick CL (2011) GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron 70:385–409
Luscher B, Keller CA (2004) Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther 102:195–221
Madsen KK, Ebert B, Clausen RP, Povl K-L, Schousboe A, White HS (2011) Selective GABA reuptake inhibitors tiagabine and EF1502 exhibit mechanistic differences to modulate the ataxia and anticonvulsant action of the extrasynaptic GABAA receptor agonist gaboxadol. J Pharm Exp Ther 338:214–219
Maguire J, Ferando I, Simonsen C, Mody I (2009) Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci 29:9592–9601
Maguire J, Mody I (2007) Neurosteroid synthesis-mediated regulation of GABAA receptors: relevance to ovarian cycle and stress. J Neurosci 27:2155–2162
Maguire J, Mody I (2008) GABAAR plasticity during pregnancy: relevance to postpartum depression. Neuron 59:207–213
Maguire JL, Stell BM, Rafizadeh M, Mody I (2005) Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 8:797–804
Maitra R, Reynolds JN (1999) Subunit dependent modulation of GABAA receptor function by neuroactive steroids. Brain Res 819:75–82
Majewska MD (1992) Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol 38:379–395
Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM (1986) Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232(4753):1004–1007
Mangan PS, Sun C, Carpenter M, Goodkin HP, Sieghart W, Kapur J (2005) Cultured hippocampal pyramidal neurons express two kinds of GABAA receptors. Mol Pharmacol 67:775–788
McCartney MR, Deeb TZ, Henderson TN, Hales TG (2007) Tonically active GABAA receptors in hippocampal pyramidal neurons exhibit constitutive GABA-independent gating. Mol Pharmacol 71:539–548
Meera P, Olsen RW, Otis TS, Wallner M (2009) Etomidate, propofol and the neurosteroid THDOC increase the GABA efficacy of recombinant α4β3δ and α4β3 GABAA receptors expressed in HEK cells. Neuropharmacology 56:155–160
Meera P, Wallner M, Otis TS (2011) Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABAA receptors. J Neurophysiol 106:2057–2064
Melcangi RC, Poletti A, Cavarretta I, Celotti F, Colciago A, Magnaghi V, Motta M, Negri-Cesi P, Martini L (1998) The 5α-reductase in the central nervous system: expression and modes of control. J Steroid Biochem Mol Biol 65:295–299
Mennerick S, He Y, Jiang X, Manion BD, Wang M, Shute A, Benz A, Evers AS, Covey DF, Zorumski CF (2004) Selective antagonism of 5α-reduced neurosteroid effects at GABAA receptors. Mol Pharmacol 65:1191–1197
Midzak A, Akula N, Lecanu L, Papadopoulos V (2011a) Novel androstanediol interacts with the mitochondrial translocator protein and controls steroidogenesis. J Biol Chem 286:9875–9887
Midzak A, Rone M, Aghazadeh Y, Culty M, Papdopoulos V (2011b) Mitochondrial protein import and the steroidogenic mitochondria. Mol Cell Endocrinol 336:70–79
Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi Z, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG et al (1999) Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci U S A 96:12905–12910
Mitchell EA, Gentet LJ, Dempster J, Belelli D (2007) GABAA and glycine receptor-mediated transmission in rat lamina II neurones: relevance to the analgesic actions of neuroactive steroids. J Physiol 583:1021–1040
Mitchell EA, Herd MB, Gunn BG, Lambert JJ, Belelli D (2008) Neurosteroid modulation of GABAA receptors: molecular determinants and significance in health and disease. Neurochem Int 52:588–595
Mitchell SJ, Silver RA (2003) Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38:433–445
Mitev YA, Darwish M, Wolf SS, Holsboer F, Almedia OFX, Patchev VK (2003) Gender differences in the regulation of 3α-hydroxysteroid dehydrogenase in rat brain and sensitivity to neurosteroid-mediated stress protection. Neuroscience 120:541–549
Mody I (2001) Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem Res 26:907–913
Mody I (2012) Plasticity of GABAA receptors relevant to neurosteroid actions. Jasper’s basic mechanisms of the epilepsies, 4th edn. NCBI, Bethesda
Mohler H, Fritschy JM, Rudolph U (2002) A new benzodiazepine pharmacology. J Pharmacol Exp Ther 300:2–8
Mortensen M, Ebert B, Wafford K, Smart TG (2010) Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J Physiol 588:1251–1268
Mortensen M, Patel B, Smart TG (2012) GABA potency at GABAA receptors found in synaptic and extrasynaptic zones. Front Neurosci 6:1–10
Mortensen M, Smart TG (2010) Extrasynaptic αβ subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol 577:841–856
Mostallino MC, Mura ML, Maciocco E, Murru L, Sanna E, Biggio G (2006) Changes in expression of the δ subunit of the GABAA receptor and in receptor function induced by progesterone exposure and withdrawal. J Neurochem 99:321–332
Mtchedlishvili Z, Lepsveridze E, Xu H, Kharlamov EA, Lu B, Kelly KM (2010) Increase of GABAA receptor-mediated tonic inhibition in dentate granule cells after traumatic brain injury. Neurobiol Dis 38:464–475
Mtchedlishvili Z, Kapur J (2003) A presynaptic action of the neurosteroid pregnenolone sulfate on GABAergic synaptic transmission. Mol Pharmacol 64:857–864
Mtchedlishvili Z, Sun CS, Harrison MB, Kapur J (2003) Increased neurosteroid sensitivity of hippocampal GABAA receptors during postnatal development. Neuroscience 118:655–666
Mukherjee J, Kretschmannova K, Gouzer G, Maric HM, Ramsden S, Tretter V, Harvey K, Davies PA, Triller A, Schindelin H et al (2011) The residence time of GABAARs at inhibitory synapses is determined by direct binding of the receptor α1 subunit to gephyrin. J Neurosci 31:14677–14687
Nagaya N, Macdonald RL (2001) Two γ2L subunit domains confer low Zn2+ sensitivity to ternary GABAA receptors. J Physiol 532:17–30
Nohria V, Tsai J, Shaw K, Rogawski MA, Pieribone VA, Farfel G (2010) Ganaxolone, in Bialer M, et al., Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X). Epilepsy Res 92:104–107
Nothdurfter C, Rammes G, Baghai TC, Schüle C, Schumacher M, Papadopoulos V, Rupprecht R (2012) TSPO (18 kDa) as a target for novel anxiolytics with a favourable side-effect profile. J Neuroendocrinol 24(1):82–92
Nusser Z, Mody I (2002) Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol 87:2624–2628
Nuñez JL, McCarthy MM (2007) Evidence for an extended duration of GABA-mediated excitation during the developing male versus female hippocampus. Dev Neurobiol 67:1879–1890
Olsen RW, Sieghart W (2009) GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56:141–148
Ortinski PI, Lu C, Takagagi K, Fu Z, Vicini S (2004) Expression of distinct α subunits of GABAA receptor regulates inhibitory synaptic strength. J Neurophysiol 92:1718–1727
Otis TS, Koninck YD, Mody I (1994) Lasting potentiation of inhibition is associated with an increased number of γ-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci U S A 91:7698–7702
Pandit S, Jeong JA, Jo JY, Cho HS, Kim DW, Kim JM, Ryu PD, Lee SY, Kim HW, Jeon BH, Park JB (2013) Dual mechanisms diminishing tonic GABAA inhibition of dentate gyrus granule cells in Noda epileptic rats. J Neurophysiol 110:95–102
Paoletti AM, Romagnino S, Contu R, Orru MM, Marotto MF, Zedda P, Lello S, Biggio G, Concas A, Melis GB (2006) Observational study on the stability of the psychological status during normal pregnancy and increased blood levels of neuroactive steroids with GABAA receptor agonist activity. Psychoneuroendocrinology 31:485–492
Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère J, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang M et al (2006) Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 27:402–409
Paul SM, Purdy RH (1992) Neuroactive steroids. FASEB J 6:2311–2322
Pavlov I, Huusko N, Drexel M, Kirchmair E, Sperk G, Pitkanen A, Walker MC (2011) Progressive loss of phasic, but not tonic, GABAA receptor-mediated inhibition in dentate granule cells in a model of post-traumatic epilepsy in rats. Neuroscience 194:208–219
Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR (2002) GABAA receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J Comp Neurol 446:179–197
Peng Z, Huang CS, Stell BM, Mody I, Houser CR (2004) Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci 24:8629–8639
Persohn E, Malherbe P, Richard JG (1992) Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp Neurol 326:193–216
Petratos S, Hirst JJ, Mendis S, Anikijenko P, Walker DW (2000) Localization of p450scc and 5α-reductase type-2 in the cerebellum of fetal and newborn sheep. Dev Brain Res 123:81–86
Petrovic M, Sedlacek M, Cais O, Horak M, Chodounska H, Vyklicky L Jr (2009) Pregnenolone sulfate modulation of N-methyl-D-aspartate receptors is phosphorylation dependent. Neuroscience 160:616–628
Pillai GV, Smith AJ, Hunt PA, Simpson PB (2004) Multiple structural features of steroids mediate subtype-selective effects on human α4β3δ GABAA receptors. Biochem Pharmacol 68:819–831
Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G (2000) GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101:815–850
Porcu P, O’Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, Morrow AL (2009) Simultaneous quantification of GABAergic 3α,5α/3α,5β neuroactive steroids in human and rat serum. Steroids 74:463–473
Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE (2006) Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol 96:846–857
Prince RJ, Simmonds MA (1992) 5 beta-pregnan-3 beta-ol-20-one, a specific antagonist at the neurosteroid site of the GABAA receptor-complex. Neurosci Lett 135:273–275
Puia G, Ducic I, Vicini S, Costa E (1993) Does neurosteroid modulatory efficacy depend on GABAA receptor subunit composition? Receptors Channels 1:135–142
Puia G, Santi M, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E (1990) Neuroactive steroids act on recombinant human GABAA receptors. Neuron 4:759–765
Purdy RH, Morrow AL, Blinn JR, Paul SM (1990) Synthesis, metabolism, and pharmacological activity of 3α-hydroxy steroids which potentiate GABA-receptor-mediated chloride ion uptake in rat cerebral cortical synaptoneurosomes. J Med Chem 33:1572–1581
Purdy RH, Morrow AL, Moore PH Jr, Paul SM (1991) Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A 88:4553–4557
Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM (1996) [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the alpha 5 subunit. Neuropharmacology 35:1331–1335
Rajasekaran K, Joshi S, Sun C, Mtchhedlishvilli Z, Kapur J (2010) Receptors with low affinity for neurosteroids and GABA contribute to tonic inhibition of granule cells in epileptic animals. Neurobiol Dis 40:490–501
Ramakrishnan L, Hess GP (2010) Mechanism of potentiation of a dysfunctional epilepsy-linked mutated GABAA receptor by a neurosteroid (3α,21-dihydroxy-5α-pregnan-20-one): transient kinetic investigations. Biochemistry 49:7892–7901
Ransom CB, Wu Y, Richerson GB (2010) Postdepolarization potentiation of GABAA receptors: a novel mechanism regulating tonic conductance in hippocampal neurons. J Neurosci 30:7672–7684
Reddy DS (2003a) Pharmacology of endogenous neuroactive steroids. Crit Rev Neurobiol 15:197–234
Reddy DS (2003b) Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions? Trends Pharmacol Sci 24(3):103–106
Reddy DS (2004a) Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3α-androstanediol and 17β-estradiol. Neuroscience 129:195–207
Reddy DS (2004b) Anticonvulsant activity of the testosterone-derived neurosteroid 3α-androstanediol. Neuroreport 15:515–518
Reddy DS (2006) Physiological role of adrenal deoxycorticosterone-derived neuroactive steroids in stress-sensitive conditions. Neuroscience 138:911–920
Reddy DS (2007) Premenstrual catamenial epilepsy. Womens Health 3:195–206
Reddy DS (2008) Mass spectrometric quantification and physiological–pharmacological activity of androgenic neurosteroids. Neurochem Int 52:541–553
Reddy DS (2009a) The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res 85:1–30
Reddy DS (2009b) Steroid hormones and sex differences in seizure susceptibility. In: Schwartzkroin P (ed) Encyclopedia of basic epilepsy research, vol 1. Academic, Oxford, pp 526–533
Reddy DS (2010) Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res 186:113–137
Reddy DS (2011) Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Front Endocrinol 2(article 38):1–11
Reddy DS (2013a) Neuroendocrine aspects of catamenial epilepsy. Horm Behav 63:254–266
Reddy DS (2013b) Role of hormones and neurosteroids in epileptogenesis. Front Cell Neurosci 7(article 115):1–20
Reddy DS, Apanites LA (2005) Anesthetic effects of progesterone are undiminished in progesterone receptor knockout mice. Brain Res 1033(1):96–101
Reddy DS, Chien B, Ramu K (2005a) A high-performance liquid chromatography-tandem mass spectrometry assay of the androgenic neurosteroid 3α-androstanediol (5α-androstan-3δ,17β-diol) in plasma. Steroids 70:879–885
Reddy DS, O’Malley BW, Rogawski MA (2005b) Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology 48:14–24
Reddy DS, Shetty AK, Apanites LA (2005c) Antiepileptic effects of the neurosteroid allopregnanolone on spontaneous recurrent seizures in the rat pilocarpine model of temporal lobe epilepsy. Epilepsia 46(suppl 8):298
Reddy DS, Carver CM, Clossen BL (2013) Accelerated limbic epileptogenesis in mice lacking delta-subunit extrasynaptic GABA-A receptors. Soc Neurosci Meeting #627.10 (N17)
Reddy DS, Gangisetty O, Briyal S (2010) Disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Neuropharmacology 59:573–581
Reddy DS, Gould J, Gangisetty O (2012) A mouse kindling model of perimenstrual catamenial epilepsy. J Pharmacol Exp Ther 341:784–793
Reddy DS, Kuruba R (2013) Experimental models of status epilepticus and neuronal injury for evaluation of therapeutic interventions. International J Mol Sci 14:18284–18318
Reddy DS, Ramanathan G (2012) Finasteride inhibits the disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Epilepsy Behav 25(1):92–97
Reddy DS, Jian K (2010) The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J Pharmacol Exp Ther 334:1031–1041
Reddy DS, Kulkarni SK (1996) Role of GABAA and mitochondrial diazepam binding inhibitor receptors in the antistress activity of neurosteroids in mice. Psychopharmacology (Berl) 128:280–292
Reddy DS, Kulkarni SK (1997) Differential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in mice. Brain Res 1997(752):61–71
Reddy DS, Kulkarni SK (1998a) The effects of neurosteroids on acquisition and retention of a modified passive-avoidance learning task in mice. Brain Res 791:108–116
Reddy DS, Kulkarni SK (1998b) Proconvulsant effects of neurosteroid pregnenolone sulfate and dehydroepiandrosterone sulfate in mice. Eur J Pharmacol 345:55–59
Reddy DS, Kulkarni SK (1999) Sex and estrous cycle-dependent changes in neurosteroid and benzodiazepine effects on food consumption and plus-maze learning behavior in rats. Pharmacol Biochem Behav 62:53–60
Reddy DS, Kulkarni SK (2000) Development of neurosteroid-based novel psychotropic drugs. Prog Med Chem 37:135–175
Reddy DS, Mohan A (2011) Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. J Neurosci 31:650–658
Reddy DS, Rogawski MA (2000a) Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther 294:909–915
Reddy DS, Rogawski MA (2000b) Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther 295:1241–1248
Reddy DS, Kim Y-H, Rogawski MA (2001) Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia 42:328–336
Reddy DS, Rogawski MA (2001) Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia 42:337–344
Reddy DS, Rogawski MA (2002) Stress-induced deoxycorticosterone-derived neurosteroids modulate GABAA receptor function and seizure susceptibility. J Neurosci 22:3795–3805
Reddy DS, Rogawski MA (2009) Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics 6:392–401
Reddy DS, Rogawski MA (2010) Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res 89:254–260
Reddy DS, Woodward R (2004) Ganaxolone: a prospective overview. Drugs Future 29:227–242
Reddy DS, Zeng YC (2007) Differential anesthetic activity of ketamine and the GABAergic neurosteroid allopregnanolone in mice lacking progesterone receptor A and B subtypes. Meth Find Exp Clin Pharmacol 29:659–664
Richerson GB, Wu Y (2003) Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol 90:1363–1374
Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K (1999) The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397:251–255
Rossi DJ, Hamann M, Attwell D (2003) Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J Physiol 548:97–110
Rudolph U, Knoflach F (2011) Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nature Rev Drug Discovery 10:685–697
Rudolph U, Mohler H (2004) Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol 44:475–498
Ruiz A, Walker MC, Fabian-Fine R, Kullmann DM (2004) Endogenous zinc inhibits GABAA receptors in a hippocampal pathway. J Neurophysiol 91:1091–1096
Rupprecht R (2003) Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology 28:139–168
Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M (2010) Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov 9:971–988
Rupprecht R, Rammes G, Eser D, Baghai TC, Schüle C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F, Uzunov V, McAllister KH, Bertaina-Anglade V, La Rochelle CD, Tuerck D, Floesser A, Kiese B, Schumacher M, Landgraf R, Holsboer F, Kucher K (2009) Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science 325(5939):490–493
Saalmann YB, Kirkcaldie MTK, Waldron S, Calford MB (2007) Cellular distribution of the GABAA receptor-modulating 3α-hydroxy, 5α-reduced pregnane steroids in the adult rat brain. J Neuroendocrinol 19:272–284
Saarelainen KS, Ranna M, Rabe H, Sinkkonen ST, Möykkynen T, Uusi-Oukari M, Linden AM, Lüdens H, Korpi ER (2008) Enhanced behavioral sensitivity to the competitive GABA agonist, gaboxadol, in transgenic mice over-expressing hippocampal extrasynaptic α6β GABAA receptors. J Neurochem 105:338–350
Sancar F, Czajkowski C (2011) Allosteric modulators induce distinct movements at the GABA-binding site interface of the GABAA receptor. Neuropharmacology 60:520–528
Sanna E, Mostallino MC, Murru L, Carta M, Talani G, Zucca S, Mura ML, Maciocco E, Biggio G (2009) Changes in expression and function of extrasynaptic GABAA receptors in the rat hippocampus during pregnancy and after delivery. J Neurosci 29:1755–1765
Santhakumar V, Hanchar HJ, Wallner M, Olsen RW, Otis TS (2006) Contributions of the GABAA receptor α6 subunit to phasic and tonic inhibition revealed by a naturally occurring polymorphism in the α6 gene. J Neurosci 26:3357–3364
Santos VR, De Castro OW, Pun RY, Hester MS, Murphy BL, Loepke AW, Garcia-Cairasco N, Danzer SC (2011) Contributions of mature granule cells to structural plasticity in temporal lobe epilepsy. Neuroscience 197:348–357
Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J (2011) Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci 31:18198–18210
Saxena NC, Macdonald RL (1994) Assembly of GABAA receptor subunits: role of the δ subunit. J Neurosci 14:7077–7086
Saxena NC, Macdonald RL (1996) Properties of putative cerebellar gamma-aminobutyric acid A receptor isoforms. Mol Pharmacol 49:567–579
Sayeed I, Stein DG (2009) Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog Brain Res 175:219–237
Scimemi A, Semyanov A, Sperk G, Kullman DM, Walker M (2005) Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci 25:10016–10024
Scholfield CN (1980) Potentiation of inhibition by general anaesthetics in neurones of the olfactory cortex in vitro. Pflugers Arch 383:249–255
Selye H (1941) Anesthetics of steroid hormones. Proc Soc Exp Biol Med 46:116–121
Sedelnikova A, Erkkila BE, Harris H, Zakharkin SO, Weiss DS (2006) Stoichiometry of a pore mutation that abolishes picrotoxin-mediated antagonism of the GABAA receptor. J Physiol 577:569–577
Semyanov A, Walker MC, Kullmann DM, Silver RA (2004) Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci 27:262–269
Serra M, Mostallino MC, Talani G, Pisu MG, Carta M, Mura ML, Floris I, Maciocco E, Sanna E, Biggio G (2006) Social isolation-induced increase in alpha and delta subunit gene expression is associated with a greater efficacy of ethanol on steroidogenesis and GABA receptor function. J Neurochem 98:122–133
Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, Biggio G (2000) Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. J Neurochem 75:732–740
Shao LR, Dudek FE (2005) Changes in mIPSCs and sIPSCs after kainite treatment: evidence for loss of inhibitory input to dentate granule cells and possible compensatory responses. J Neurophysiol 94:952–960
Shen H, Gon QH, Yuan M, Smith SS (2005) Short-term steroid treatment increases δ GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology 49:573–586
Shu HJ, Bracamontes J, Taylor A, Wu K, Eaton MM, Gustav A, Manion B, Evers AS, Krishnan K, Covey DF et al (2012) Characteristics of concatemeric GABAA receptors containing α4/δ subunits expressed in Xenopus oocytes. Br J Pharmacol 165:2228–2243
Shu HJ, Eisenman LN, Jinadasa D, Covey DF, Zorumski CF, Mennerick S (2004) Slow actions of neuroactive steroids at GABAA receptors. J Neurosci 24:6667–6675
Sieghart W (1995) Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharm Rev 47:181–234
Sieghart W (2006) Structure, pharmacology, and function of GABAA receptor subtypes. Adv Pharmacol 54:231–263
Sieghart W, Sperk G (2002) Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem 2:795–816
Smith AJ, Alder L, Silk J, Adkins C, Fletcher AE, Scales T, Kerby J, Marshall G, Wafford KA, McKernan RM et al (2001) Effect of α subunit on allosteric modulation of ion channel function in stably expressed human recombinant γ-aminobutyric acidA receptors determined using 36Cl ion flux. Mol Pharmacol 59:1108–1118
Smith SS, Gong QH (2005) Neurosteroid administration and withdrawal alter GABAA receptor kinetics in CA1 hippocampus of female rats. J Physiol 564:421–436
Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X (1998) GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392:926–929
Smith SS, Shen H, Gong QH, Zhou X (2007) Neurosteroid regulation of GABAA receptors: focus on the α4 and δ subunits. Pharmacol Ther 116:58–76
Snead OC 3rd (1998) Ganaxolone, a selective, high-affinity steroid modulator of the γ-aminobutyric acid-A receptor, exacerbates seizures in animal models of absence. Ann Neurol 44:688–691
Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W (1997) GABAA receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience 80:987–1000
Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW (2002) Behavior and physiology of mice lacking the GABAA receptor δ subunit. Epilepsia 43(suppl 5):3–8
Spigelman I, Li Z, Liang J, Cagetti E, Samzadeh S, Mihalek RM, Homanics GE, Olsen RW (2003) Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABAA receptor δ subunit. J Neurophysiol 90:903–910
Stell BM, Brickley SG, Tang CY, Farrant M, Mody I (2003) Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci U S A 100:14439–14444
Stell BM, Mody I (2002) Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci 22:RC223
Stoffel-Wagner B (2001) Neuroactive steroid metabolism in the human brain. Eur J Endocrinol 145:669–679
Stoffel-Wagner B, Beyenburg S, Watzka MS, Blumcke I, Bauer J, Schramm J, Bidlingmaier F, Elger CE (2000) Expression of 5α-reductase and 3α-hydroxysteroid oxidoreductase in the hippocampus of patients with chronic temporal lobe epilepsy. Epilepsia 41:140–147
Stoffel-Wagner B, Watzka M, Steckelbroeck S, Ludwig M, Clusmann H, Bidlingmaier F, Casarosa E, Luisi S, Elger CE, Beyenburg S (2003) Allopregnanolone serum levels and expression of 5α-reductase and 3α-hydroxysteroid dehydrogenase isoforms in hippocampal and temporal cortex of patients with epilepsy. Epilepsy Res 54:11–19
Stórustovu S, Ebert B (2003) Gaboxadol: in vitro interaction studies with benzodiazepines and ethanol suggest functional selectivity. Eur J Pharmacol 467:49–56
Stórustovu S, Ebert B (2006) Pharmacological characterization of agonists at δ-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharm Exp Ther 316:1351–1359
Strömberg J, Bäckström T, Lungren P (2005) Rapid non-genomic effects of glucocorticoid metabolites and neurosteroids on the γ-aminobutyric acid-A receptor. Eur J Neurosci 21:2083–2088
Succol F, Fiumelli H, Benfenati F, Cancedda L, Barberis A (2012) Intracellular chloride concentration influences the GABAA receptor subunit composition. Nat Commun 3:738
Sun C, Sieghart W, Kapur J (2004) Distribution of α1, γ2, and δ subunits of GABAA receptors in hippocampal granule cells. Brain Res 1029:207–216
Sun C, Mtchedlishvili Z, Erisir A, Kapur J (2007) Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the α4 subunit of GABAA receptors in an animal model of epilepsy. J Neurosci 27:12641–12650
Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS (2002) Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci 5:721–722
Suryanarayanan A, Liang J, Meyer EM, Lindemeyer AK, Chandra D, Homanics GE, Sieghart W, Olsen RW, Spigelman I (2011) Subunit compensation and plasticity of synaptic GABAA receptors induced by ethanol in α4 subunit knockout mice. Front Neurosci 5:110
Szyndler J, Maciejak P, Turzynska D, Sobolewska A, Lehner M, Taracha E, Walkowiak J, Skorzewska A, Wislowska-Stanek A, Hamed A et al (2008) Changes in the concentration of amino acids in the hippocampus of pentylenetetrazole-kindled rats. Neurosci Lett 439:245–249
Tang X, Hernandez CC, Macdonald RL (2010) Modulation of spontaneous and GABA-evoked tonic α4β3δ and α4β3γ2L GABAA receptor currents by protein kinase A. J Neurophysiol 103:1007–1019
Tao W, Higgs MH, Spain WJ, Ransom CB (2013) Postsynaptic GABAB receptors enhance GABAA receptor function in dentate gyrus granule cells. J Neurosci 33:3738–3743
Teschemacher A, Kasparov S, Kravitz EA, Rahamimoff R (1997) Presynaptic action of the neurosteroid pregnenolone sulfate on inhibitory transmitter release in cultured hippocampal neurons. Brain Res 772:226–232
Thomas P, Mortensen M, Hosie AM, Smart TG (2005) Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat Neurosci 8:889–897
Tsuda M, Suzuki T, Misawa M (1997) Modulation of the decrease in the seizure threshold of pentylenetetrazole in diazepam-withdrawn mice by the neuroactive steroid 5α-pregnan-3α,21-diol-20-one (alloTHDOC). Addiction Biol 2:455–460
Tuveri A, Paoletti AM, Orru M, Melis GB, Marotto MF, Zedda P, Marrosu F, Sogliano C, Marra C, Biggio G (2008) Reduced serum level of THDOC, an anticonvulsant steroid, in women with perimenstrual catamenial epilepsy. Epilepsia 49:1221–1229
Twyman RE, Macdonald RL (1992) Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol 456:215–245
Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH (1997) Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J Neurosci 17:625–634
Uusi-Oukari M, Korpi ER (2010) Regulation of GABAA receptor subunit expression by pharmacological agents. Pharmacol Rev 62:97–135
Vardya I, Hoestgaard-Jensen K, Nieto-Gonzalez JL, Dósa Z, Boddum K, Holm MM, Wolinsky TD, Jones KA, Dalby NO, Ebert B, Jensen K (2012) Positive modulation of δ-subunit containing GABA(A) receptors in mouse neurons. Neuropharmacology 63:469–479
Vithlani M, Terunuma M, Moss SJ (2011) The dynamic modulation of GABAA receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev 91:1009–1022
Wafford KA, Ebert B (2006) Gaboxadol—a new awakening in sleep. Curr Opin Pharmacol 6:30–36
Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ (1996) Functional characterization of human γ-aminobutyric acidA receptors containing the α4 subunit. Mol Pharmacol 50:670–678
Walls AB, Nilsen LH, Eyjolffson EM, Vestergaard HT, Hansen ST, Schousboe A, Sonnewald U, Waagepetersen HS (2010) GAD65 is essential for synthesis of GABA destined for tonic inhibition regulating epileptiform activity. J Neurochem 115:1398–1408
Wang M, He Y, Eisenman LN, Fields C, Zeng CM, Mathews J, Benz A, Fu T, Zorumski E, Steinbach JH et al (2002) 3beta-Hydroxypregnane steroids are pregnenolone sulfate-like GABAA receptor antagonists. J Neurosci 22:3366–3375
Wang MD, Rahman M, Zhu D, Johansson IM, Bäckström T (2007) 3β-Hydroxysteroids and pregnenolone sulfate inhibit recombinant rat GABAA receptor through different channel property. Eur J Pharmacol 557:124–131
Wang M, Seippel L, Purdy RH, Bäckström T (1996) Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5alpha-pregnan-20-one. J Clin Endocrinol Metab 81:1076–1082
Wei W, Zhang N, Peng Z, Houser CR, Mody I (2003) Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci 23:10650–10661
Wieland S, Belluzzi JD, Stein L, Lan NC (1995) Comparative behavioral characterization of the neuroactive steroids 3α-OH, 5α-pregnan-20-one and 3α-OH,5β-pregnan-20-one in rodents. Psychopharmacology (Berl) 118:65–71
Williams CA, Bell SV, Jenkins A (2010) A residue in loop 9 of the β2-subunit stabilizes the closed state of the GABAA receptor. J Biol Chem 285:7281–7287
Williamson J, Mtchedlishvili Z, Kapur J (2004) Characterization of the convulsant action of pregnenolone sulfate. Neuropharmacology 46:856–864
Wilson MA, Biscardi R (1997) Influence of gender and brain region on neurosteroid modulation of GABA responses in rat. Life Sci 60:1679–1691
Wisden W, Herb A, Wieland H, Keinӓnen LH, Seeburg PH (1991) Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor α4 subunit. FEBS 289:227–230
Wisden W, Laurie DJ, Monyer H, Seeburg PH (1992) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. telencephalon, diencephalon, mesencephalon. J Neurosci 12:1040–1062
Wohlfarth KM, Bianchi MT, Macdonald RL (2002) Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci 22:1541–1549
Wojtowicz T, Lebida K, Mozrzymas JW (2008) 17beta-estradiol affects GABAergic transmission in developing hippocampus. Brain Res 1241:7–17
Wu FS, Gibbs TT, Farb DH (1991) Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol 40:333–336
Wu X, Gangisetty O, Carver CM, Reddy DS (2013) Estrous cycle regulation of extrasynaptic δ-containing GABAA receptor-mediated tonic inhibition and limbic epileptogenesis. J Pharmacol Exp Ther 346:146–160
Wu X, Wu Z, Ning G, Guo Y, Ali R, Macdonald RL, De Blas AL, Luscher B, Chen G (2012) GABAA receptor alpha subunits play a direct role in synaptic versus extrasynaptic targeting. J Biol Chem 287:27417–27430
Wu Y, Wang W, Diez-Sampedro A, Richerson GB (2007) Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron 56:851–865
Wu Y, Wang W, Richerson GB (2006) The transmembrane sodium gradient influences ambient GABA concentration by altering the equilibrium of GABA transporters. J Neurophysiol 96:2425–2436
Wulff P, Goetz T, Leppӓ E, Linden A-M, Renzi M, Swinny JD, Vekovischeva OY, Sieghart W, Somogyi P, Korpi ER (2007) From synapse to behavior: rapid modulation of defined neuronal types with engineered GABAA receptors. Nat Neurosci 10:923–929
Yeung JYT, Canning KJ, Zhu G, Pennefather P, Macdonald JF, Orser BA (2003) Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol 63:2–8
You H, Dunn SM (2007) Identification of a domain in the δ subunit (S238-V264) of the α4β3δ GABAA receptor that confers high agonist sensitivity. J Neurochem 103:1092–1101
Yu W, Jiang M, Miralles CP, Li RW, Chen G, de Blas AL (2007) Gephyrin clustering is required for the stability of GABAergic synapses. Mol Cell Neurosci 36:484–500
Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C (2006) Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res 1099:73–81
Zhan RZ, Nadler JV (2009) Enhanced tonic GABA current in normotropic and hilar ectopic dentate granule cells after pilocarpine-induced status epilepticus. J Neurophysiol 102:670–681
Zhang N, Wei W, Mody I, Houser CR (2007) Altered localization of GABAA receptor subunits on dentate granule cell dendrites influence tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci 27:7520–7531
Zheleznova NN, Sedelnikova A, Weiss DS (2008) α1β2δ, a silent GABAA receptor: recruitment by tracazolate and neurosteroids. Br J Pharmacol 153:1062–1071
Zheleznova NN, Sedelnikova A, Weiss DS (2009) Function and modulation of δ-containing GABAA receptors. Psychoneuroendocrinology 34S:S67–S73
Zhu WJ, Vicini S (1997) Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. J Neurosci 17:4022–4031
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke Grants NS051398, NS071597, and NS076426 (to D.S.R.). We thank Drs. William Griffith and Gerald Frye for comments on the manuscript. The authors would like to thank Xin Wu for technical help with illustrations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carver, C.M., Reddy, D.S. Neurosteroid interactions with synaptic and extrasynaptic GABAA receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology 230, 151–188 (2013). https://doi.org/10.1007/s00213-013-3276-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3276-5