Abstract
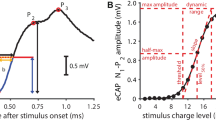
After severe hair cell loss, secondary degeneration of spiral ganglion cells (SGCs) is observed—a gradual process that spans years in humans but only takes weeks in guinea pigs. Being the target for cochlear implants (CIs), the physiological state of the SGCs is important for the effectiveness of a CI. For assessment of the nerve’s state, focus has generally been on its response threshold. Our goal was to add a more detailed characterization of SGC functionality. To this end, the electrically evoked compound action potential (eCAP) was recorded in normal-hearing guinea pigs and guinea pigs that were deafened 2 or 6 weeks prior to the experiments. We evaluated changes in eCAP characteristics when the phase duration (PD) and inter-phase gap (IPG) of a biphasic current pulse were varied. We correlated the magnitude of these changes to quantified histological measures of neurodegeneration (SGC packing density and SGC size). The maximum eCAP amplitude, derived from the input–output function, decreased after deafening, and increased with both PD and IPG. The eCAP threshold did not change after deafening, and decreased with increasing PD and IPG. The dynamic range was wider for the 6-weeks-deaf animals than for the other two groups. Excitability increased with IPG (steeper slope of the input–output function and lower stimulation level at the half-maximum eCAP amplitude), but to a lesser extent for the deafened animals than for normal-hearing controls. The latency was shorter for the 6-weeks-deaf animals than for the other two groups. For several of these eCAP characteristics, the effect size of IPG correlated well with histological measures of degeneration, whereas effect size of PD did not. These correlations depend on the use of high current levels, which could limit clinical application. Nevertheless, their potential of these correlations towards assessment of the condition of the auditory nerve may be of great benefit to clinical diagnostics and prognosis in cochlear implant recipients.










Similar content being viewed by others
References
Abbas PJ, Miller CA (2004) Biophysics and physiology. In: Zeng F-G, Popper AN, Fay RR (eds) Cochlear implants: auditory prostheses and electric hearing. Springer Handbook of Auditory Research. Springer, New York, pp 149–212
Agterberg MJH, Versnel H, De Groot JCMJ, Smoorenburg GF, Albers FWJ, Klis SFL (2008) Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear Res 244:25–34
Agterberg MJH, Versnel H, Van Dijk LM, De Groot JCMJ, Klis SFL (2009) Enhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigs. J Assoc Res Otolaryngol 10:355–367
Cappaert NLM, Ramekers D, Martens HCF, Wadman WJ (2013) Efficacy of a new charge-balanced biphasic electrical stimulus in the isolated sciatic nerve and the hippocampal slice. Int J Neural Syst 23:1250031
Carlyon RP, van Wieringen A, Deeks JM, Long CJ, Lyzenga J, Wouters J (2005) Effect of inter-phase gap on the sensitivity of cochlear implant users to electrical stimulation. Hear Res 205:210–224
Coggeshall RE, Lekan HA (1996) Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol 364:6–15
Ernfors P, Van De Water T, Loring J, Jaenisch R (1995) Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron 14:1153–1164
Ernfors P, Duan ML, ElShamy WM, Canlon B (1996) Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med 2:463–467
Fayad JN, Linthicum FH Jr (2006) Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope 116:1310–1320
Fransson A, Maruyama J, Miller JM, Ulfendahl M (2010) Post-treatment effects of local GDNF administration to the inner ears of deafened guinea pigs. J Neurotrauma 27:1745–1751
Frijns JHM, Briaire JJ, Grote JJ (2001) The importance of human cochlear anatomy for the results of modiolus-hugging multichannel cochlear implants. Otol Neurotol 22:340–349
Frijns JHM, de Snoo SL, ten Kate JH (1996) Spatial selectivity in a rotationally symmetrical model of the electrically stimulated cochlea. Hear Res 95:33–48
Fritzsch B, Pirvola U, Ylikoski J (1999) Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell Tissue Res 295:369–382
Glueckert R, Bitsche M, Miller JM, Zhu Y, Prieskorn DM, Altschuler RA, Schrott-Fischer A (2008) Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor. J Comp Neurol 507:1602–1621
Grill WM, Norman SE, Bellamkonda RV (2009) Implanted neural interfaces: biochallenges and engineered solutions. Annu Rev Biomed Eng 11:1–24
Hall RD (1990) Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked auditory brainstem response. Hear Res 45:123–36
Kim JR, Abbas PJ, Brown CJ, Etler CP, O’Brien S, Kim LS (2010) The relationship between electrically evoked compound action potential and speech perception: a study in cochlear implant users with short electrode array. Otol Neurotol 31:1041–1048
Koles ZJ, Rasminsky M (1972) A computer simulation of conduction in demyelinated nerve fibres. J Physiol 227:351–364
Leake PA, Hradek GT, Hetherington AM, Stakhovskaya O (2011) Brain-derived neurotrophic factor promotes cochlear spiral ganglion cell survival and function in deafened, developing cats. J Comp Neurol 519:1526–1545
Limón A, Pérez C, Vega R, Soto E (2005) Ca2+-activated K+-current density is correlated with soma size in rat vestibular-afferent neurons in culture. J Neurophysiol 94:3751–3761
Macherey O, Carlyon RP, van Wieringen A, Deeks JM, Wouters J (2008) Higher sensitivity of human auditory nerve fibers to positive electrical currents. J Assoc Res Otolaryngol 9:241–251
Marquardt DW (1963) An algorithm for least-squares estimation of nonlinear parameters. J Soc Ind Appl Math 11:431–441
Maruyama J, Miller JM, Ulfendahl M (2008) Glial cell line-derived neurotrophic factor and antioxidants preserve the electrical responsiveness of the spiral ganglion neurons after experimentally induced deafness. Neurobiol Dis 29:14–21
McKay CM, Henshall KR (2003) The perceptual effects of interphase gap duration in cochlear implant stimulation. Hear Res 181:94–99
Miller AL, Smith DW, Pfingst BE (1999) Across-species comparisons of psychophysical detection thresholds for electrical stimulation of the cochlea: II. Strength–duration functions for single, biphasic pulses. Hear Res 135:47–55
Miller CA, Abbas PJ, Rubinstein JT, Robinson BK, Matsuoka AJ, Woodworth G (1998) Electrically evoked compound action potentials of guinea pig and cat: responses to monopolar, monophasic stimulation. Hear Res 119:142–154
Miller CA, Woodruff KE, Pfingst BE (1995) Functional responses from guinea pigs with cochlear implants. I. Electrophysiological and psychophysical measures. Hear Res 92:85–99
Moon AK, Zwolan TA, Pfingst BE (1993) Effects of phase duration on detection of electrical stimulation of the human cochlea. Hear Res 67:166–178
Neustetter C, Zangerl M, Spitzer P, Zierhofer C (2012) In-vitro characterization of a cochlear implant system for recording of evoked compound action potentials. Biomed Eng Online 11:22
Prado-Guitierrez P, Fewster LM, Heasman JM, McKay CM, Shepherd RK (2006) Effect of interphase gap and pulse duration on electrically evoked potentials is correlated with auditory nerve survival. Hear Res 215:47–55
Ramekers D, Versnel H, Grolman W, Klis SFL (2012) Neurotrophins and their role in the cochlea. Hear Res 288:19–33
Rattay F, Lutter P, Felix H (2001) A model of the electrically excited human cochlear neuron. I. Contribution of neural substructures to the generation and propagation of spikes. Hear Res 153:43–63
Richardson RT, O’Leary S, Wise A, Hardman J, Clark G (2005) A single dose of neurotrophin-3 to the cochlea surrounds spiral ganglion neurons and provides trophic support. Hear Res 204:37–47
Shepherd RK, Coco A, Epp SB, Crook JM (2005) Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol 486:145–158
Shepherd RK, Javel E (1997) Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res 108:112–144
Shepherd RK, Javel E (1999) Electrical stimulation of the auditory nerve: II. Effect of stimulus waveshape on single fibre response properties. Hear Res 130:171–188
Sly DJ, Heffer LF, White MW, Shepherd RK, Birch MG, Minter RL, Nelson NE, Wise AK, O’Leary SJ (2007) Deafness alters auditory nerve fibre responses to cochlear implant stimulation. Eur J Neurosci 26:510–522
Spoendlin H (1975) Retrograde degeneration of the cochlear nerve. Acta Otolaryngol 79:266–275
Spoendlin H (1984) Factors inducing retrograde degeneration of the cochlear nerve. Ann Otol Rhinol Laryngol Suppl 112:76–82
Stypulkowski PH, van den Honert C (1984) Physiological properties of the electrically stimulated auditory nerve. I. Compound action potential recordings. Hear Res 14:205–223
Van den Honert C, Mortimer JT (1979) The response of the myelinated nerve fiber to short duration biphasic stimulating currents. Ann Biomed Eng 7:117–125
Van Loon MC, Ramekers D, Agterberg MJH, De Groot JCMJ, Grolman W, Klis SFL, Versnel H (2013) Spiral ganglion cell morphology in guinea pigs after deafening and neurotrophic treatment. Hear Res 298:17–26
Versnel H, Agterberg MJH, De Groot JCMJ, Smoorenburg GF, Klis SFL (2007) Time course of cochlear electrophysiology and morphology after combined administration of kanamycin and furosemide. Hear Res 231:1–12
Webster M, Webster DB (1981) Spiral ganglion neuron loss following organ of Corti loss: a quantitative study. Brain Res 212:17–30
West BA, Brummett RE, Himes DL (1973) Interaction of kanamycin and ethacrynic acid. Severe cochlear damage in guinea pigs. Arch Otolaryngol 98:32–37
Westen AA, Dekker DM, Briaire JJ, Frijns JHM (2011) Stimulus level effects on neural excitation and eCAP amplitude. Hear Res 280:166–176
Xu HX, Kim GH, Snissarenko EP, Cureoglu S, Paparella MM (2012) Multi-channel cochlear implant histopathology: are fewer spiral ganglion cells really related to better clinical performance? Acta Otolaryngol 132:482–490
Ylikoski J, Wersall J, Bjorkroth B (1974) Degeneration of neural elements in the cochlea of the guinea pig after damage to the organ of corti by ototoxic antibiotics. Acta Otolaryngol Suppl 326:23–41
Zilberstein Y, Liberman MC, Corfas G (2012) Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J Neurosci 32:405–410
Acknowledgments
The authors would like to thank Ferry Hendriksen for histological processing, René van de Vosse for technical support, and Roland Hessler at MED-EL, Innsbruck, for the electrode arrays. This work was supported by MED-EL GmbH, Innsbruck, Austria.
Conflict of Interest Disclosure Statement
Wilko Grolman received unrestrictive research grants from Cochlear Ltd., MED-EL GmbH, and Advanced Bionics. No competing interests declared by the other authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramekers, D., Versnel, H., Strahl, S.B. et al. Auditory-Nerve Responses to Varied Inter-Phase Gap and Phase Duration of the Electric Pulse Stimulus as Predictors for Neuronal Degeneration. JARO 15, 187–202 (2014). https://doi.org/10.1007/s10162-013-0440-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-013-0440-x