Abstract
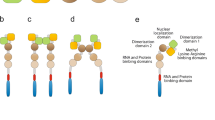
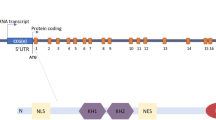
Fragile x syndrome (FXS) is the most common form of inherited mental retardation disease. This is caused due to expansion of CGG triplet in 5′-untranslated region of fragile x mental retardation 1 (FMR-1) gene. In most of the cases, abnormally large size of the CGG repeat (>200) undergoes hypermethylation, which in turn silences the FMR-1 gene causing thereby complete lack of its protein product called fragile x mental retardation protein (FMRP). Lack of FMRP due to gene silencing or production of faulty protein due to point mutation in KH2 domain of FMRP alters the translational process in neurons and leads to expression of mental retardation phenotype on the patients. The FMRP is expressed ubiquitously in all tissues; however, it is predominantly expressed in neurons and testis. It possesses heterogeneity and is found in many isoforms due to alternative splicing of the FMR-1 transcript. Based on our data from the Western-, slot-, Northern blotting and immunohistochemical studies, we report here the down regulation of Fmr-1 gene and FMRP in mice brain in age-dependent manner. The present finding is important in respect to FMRP-dependent various brain functions i.e., learning, memory, cognition etc. that decrease with advancing age.




Similar content being viewed by others
References
Zalfa F, Achsel T, Bagni C (2006) mRNPs, polysomes or granules: FMRP in neuronal protein synthesis. Curr Opin Neurobiol 16:1–5
Garber K, Smith KT, Reines D, Warren ST (2006) Transcription, translation and fragile x syndrome. Curr Opin Genet Dev 16:1–6
O’Donnell WT, Warren ST (2002) A decade of molecular studies of fragile x syndrome. Annu Rev Neurosci 25:315–338
Siomi H, Matunic MJ, Michael WM, Dreyfuss G (1993a) The pre-mRNA binding K protein contains a novel evolutionarily conserved motif. Nucleic Acid Res 21:1193–1198
Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G (1993b) The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell 74:291–298
Bardoni B, Schenick A, Mandel JL (2001) The fragile X mental retardation protein. Brain Res Bull 56:375–382
De Boulle K, Verkerk AJMH, Reyniers E, Vits L, Hendrickx J, Van Roy B, Van Den Bos F, de Graaff E, Oostra BA, Willems PJ (1993) A point mutation in the FMR1 gene associated with fragile X mental retardation. Nat Genet 3:31–35
Khandjian EW, Fortin A, Thibodeau A, Tremblay S, Cote F, Devys D, Mandel J- L, Rousseau F (1995) A heterogeneous set of FMR1 proteins is widely distributed in mouse tissues and is modulated in cell culture. Hum Molec Genet 4:783–789
Abitbol M, Menini C, Delezoide AL, Rhyner T, Vekemans M, Mallet J (1993) Nucleus basalis magnocellularis and hippocampus are the major sites of FMR-1 expression in the human fetal brain. Nat Genet 4:147–153
Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL (1993) The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet 4:335–340
Hinds HL, Ashley CT, Sutcliffe JS, Nelson DL, Warren ST, Housman DE, Schalling M (1993) Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome. Nat Genet 3:36–43
Wang H, Ku1 L, Osterhout DJ, Li W, Ahmadian A, Liang Z, Feng Y (2004) Developmentally-programmed FMRP expression in oligodendrocytes: a potential role of FMRP in regulating translation in oligodendroglia progenitors. Hum Mol Genet 13:79–89
Maroui R, Huot ME, Tremblay S, Filion C, Labelle Y, Khandjian EW (2002) Trapping of messenger RNA by fragile X mental retardation protein into cytoplasmic granules induces translation repression. Hum Mol Genet 11:3007–3017
Carthew RW (2002) RNA interference: the fragile X syndrome connection. Curr Biol 12:R852–R854
Kalidas S, Smith DP (2004) Functional Genomics, fragile x syndrome and RNA interference. Arch Neurol 60:1197–1200
Gabus C, Mazroui R, Tremblay S, Khandjian EW, Darlix J (2004) The fragile X mental retardation protein has nucleic acid chaperone properties. Nucleic Acid Res 32:2129–2137
Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U (2001) Evidence that fragile-x-mental retardation protein is negative regulator of translation. Hum Mol Genet 10:329–338
Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ (2001) Synaptic regulation of protein synthesis and the fragile-x-protein. Proc Natl Acad Sci USA 98:7101–7106
Bardoni B, Davidovic L, Bensaid M, Khandjian EW (2006) The fragile X syndrome: exploring its molecular basis and seeking a treatment. Expert Rev Mol Med 8:1–16
Katharina B, Segal M (2000) FMRP involvement in formation of synapses among cultured hippocampal neurons. Cereb Cortex 10:1045–1052
Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ (2004) Metabotropic glutamate receptor activation regulates fragile x mental retardation protein and FMR1 mRNA coalization differentially in dendrites and at synapes. J Neurosci 24:26–4865
Visootsak J, Warren ST, Anido A, Graham JM (2005) Fragile X syndrome: an update and review for the primary pediatrician. Clin Paediatr 44:371–381
Wong TP (2002) Aging of the cerebral cortex. MJM 6:104–113
Kenneson A, Zhang F, Hagdorn CH, Warren ST (2001) Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet 10:1449–1454
Tassone F, Hagerman RJ, Taylor AK, Hagerman PJ (2001) A majority of fragile X males with methylated, full mutation alleles have significant levels of FMR1 messenger RNA. J Med Genet 38:453–456
Jacquemont S, Farzin F et al (2004) Aging in individuals with the FMR1 mutation. Am J Ment Retard 109:154–164
Welt CK, Smith PC, Taylor AE (2004) Evidence of early ovarian aging in fragile X premutation carriers. J Clin Endocrinol Metab 89:4569–4574
Kim SK, Ro JY, Kemp BL, Lee JS, Kwon TJ, Fong KM, Sekido Y, Minna JD, Hong WK, Mao L (1997) Identification of three distinct tumor suppressor loci on the short arm of chromosome 9 in small cell lung cancer. Cancer Res 57:400–403
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Laemmli UK (1970) Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature 227:680–685
Shambrook J, Russel DW (2001) Molecular cloning: a laboratory manual, 3rd edn., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
De Diego Otero Y, Severijnen LA, van Cappellen G, Schrier M, Oostra B, Willemsen R (2002) Transport of fragile X mental retardation protein via granules in neurites of PC12 cells. Mol Cell Biol 22:8332–8341
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl (1995) Short protocols in Molecular Biology.John Wiley and Sons, New York
Hsu JC (1996) Multiple Comparisons: Theory and Methods, 1st edn. Chapman and Hall/CRC, New York
Lu R, Wang H, Liang Z, Ku L, O’Donnell WT, Li W, Warren ST, Feng Y, (2004) The fragile x protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci USA 101:15201–15206
Ma D, Nothias F, Boyne LJ, Fischer I (1997) Differential regulation of microtubule-associated protein 1B (MAP1B) in rat CNS and PNS during development. J Neurosci Res 49:319–332
Bear MF, Huber KM, Warren ST (2004) The mGluR theory of fragile X mental retardation. Trends Neurosci 27:370–376
Gong R, Park CS, Abbassi NR, Tang S (2006) Roles of glutamate receptors and the mtor signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem 1–22. (http://www.jbc.org/cgi/doi/10.1074/jbc.M512524200)
Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler JI, Greenough WT (1997) Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci USA 94:5401–5404
Huber KM, Gallagher SM, Warren ST, Bear MF (2002) Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA 99:7746–7750
Larson J, Jessen RE, Kim D, Ananda-Kriiya S, Hoffmann J (2005) Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking FMRP. J Neurosci 25:9460–9469
Miller G (2006) Fragile X’s unwelcome relative. Science 312:518–521
Huber KM (2006) The fragile X–cerebellum connection. Trends Neurosci 29:183–185
Gruss M, Braun K (2004) Age- and region specific imbalances of amino acids and monoamine metabolism in limbic regions of female Fmr1 knock-out mice. Neurochem Internat 45:81–88
Acknowledgement
The authors acknowledge Prof. MS Kanungo and Prof. MK Thakur for providing support and thank Profs. EW Khandjian, Canada, U Fischer, Germany and J-L Mandel, France for donating anti-FMRP antibody. The financial assistance from CAS in Zoology, Banaras Hindu University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, K., Gaur, P. & Prasad, S. Fragile x mental retardation (Fmr-1) gene expression is down regulated in brain of mice during aging. Mol Biol Rep 34, 173–181 (2007). https://doi.org/10.1007/s11033-006-9032-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-006-9032-8