Abstract
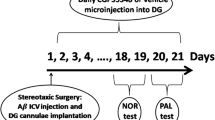
The possible link between amnesia induced by central administration of β-amyloid (25–35) (Aβ(25–35)) and neurodegenerative changes in the hippocampus was studied. Male Wistar rats received single intracerebroventricular injections of Aβ(25–35) at a dose of 15 nmoles and one month later were trained in an eight-arm radial maze. Training was followed by histological assessment of the state of the hippocampus on brain sections stained with hematoxylin and eosin. Aβ(25–35) induced impairments in long-term (reference) and working memory on testing in the maze. There was a moderate reduction in the number of neurons in hippocampal field CA1; there was no change in the number of cells in field CA3. The numbers of errors made by the animals on testing in the maze were found to correlate negatively with the numbers of nerve cells in hippocampal field CA1. Thus, this is the first demonstration that impairments of learning and memory induced by single doses of Aβ(25–35) are specifically associated with neurodegenerative changes in hippocampal field CA1 in rats.
Similar content being viewed by others
REFERENCES
O. S. Vinogradova, The Hippocampus and Memory [in Russian], Nauka, Moscow (1975).
P. V. Simonov, The Motivated Brain [in Russian], Nauka, Moscow (1987).
E. N. Sokolov, N. I. Nezlina, V. B. Polyanskii, and D. V. Evtikhin, “The orientational reflex: the targeting reflex’ and the ‘projector of attention,’” Zh. Vyssh. Nerv. Deyat., 51, No.4, 421–437 (2001).
M. Yu. Stepanovich, N. A. Lazareva, M. V. Onufriev, O. S. Mitrokhina, Yu. V. Moiseeva, and N. V. Gulyaeva, “Effects of administration of fragment (25–35) of beta-amyloid peptide on behavior in rats,” Zh. Vyssh. Nerv. Deyat., 47, No.3, 597–600 (1997).
M. Yu. Stepanovich, Yu. V. Moiseeva, and N. V. Gulyaeva, “‘Injection’ models of Alzheimer's disease: oxidative stress in the mechanism of toxicity of AF64 and β-amyloid peptide in rodents,” Neirokhimiya, 19, No.3, 165–175 (2002).
M. Yu. Stepanichev, N. V. Onufriev, O. S. Mitrokhina, Yu. V. Moiseeva, N. A. Lazareva, I. V. Viktorov, and N. V. Gulyaeva, “Neurochemical, behavioral and neuromorphological effects of central administration of beta-amyloid peptide (25–35) in rats,” Neirokhimiya, 17, No.4, 291–306 (2000).
E. Abe, F. Casamenti, L. Giovanelli, S. Scali, and G. Pepeu, “Administration of amyloid beta-peptides into the medial septum on rats decreases acetylcholine releaser from hippocampus in vivo,” Brain Res., 636, 162–164 (1994).
A. Baddeley, “The fractionation of working memory,” Proc. Natl. Acad. Sci. USA, 93, 13468–13472 (1993).
H. Braak and E. Braak, “Neuropathological staging of Alzheimer-related changes,” Acta Neuropathol., 82, 239–259 (1991).
S. Delobette, A. Privat, and T. Maurice, “In vitro aggregation facilitates beta-amyloid peptide (25–35)-induced amnesia in the rat,” Eur. J. Pharmacol., 319, 1–4 (1997).
L. E. Jarrard, “What does the hippocampus really do?” Behav. Brain Res., 71, 1–10 (1995).
R. Katzman, “Epidemiology of Alzheimer's disease,” Neurobiol. Aging, 21, 51–56 (2000).
Y. Kiyota, M. Miyamoto, and A. Nagaoka, “Relationship between brain damage and memory impairment in rats exposed to transient ischemia,” Brain Res., 538, 295–302 (1991).
T. Maurice, B. P. Lockhart, and A. Privat, “Amnesia induced in mice by centrally administered beta-amyloid peptides involves cholinergic dysfunction,” Brain Res., 706, 181–193 (1996).
M. P. McDonald and Y. B. Overmeier, “Present imperfect: a critical review of animal models of mnemonic impairments in Alzheimer's disease,” Neurosci. Biobehav. Rev., 22, 99–120 (1998).
S. Nakamura, M. Murayama, T. Noshita, H. Annoura, and T. Ohno, “Progressive brain dysfunction following intracerebroventricular infusion of beta1-42-amyloid peptide,” Brain Res., 912, 128–136 (2001).
A. Nelson, A. Lebessi, P. Sowinski, and H. Hodges, “Comparison of effects of global cerebral ischaemia on spatial earning in the standard and radial water maze: relationship of hippocampal damage to performance,” Behav. Brain Res., 85, 93–115 (1997).
A. Nitta, A. Iton, T. Hasegawa, and T. Nabeshima, “β-Amyloid-protein-induced Alzheimer's disease animal model,” Neurosci. Lett., 28, 63–66 (1994).
J. A. Nunn, E. LePeillet, C. A. Netto, H. Hodges, J. A. Gray, and B. S. Meldrum, “Global ischemia: hippocampal pathology and spatial deficits in the water maze,” Behav. Brain Res., 62, 41–54 (1994).
A. Olariu, M. H. Tran, K. Yamada, M. Mizuno, V. Hefco, and T. Nabashima, “Memory deficits and increased emotionality induced by β-amyloid (25–35) are correlated with the reduced acetylcholine release and altered phorbol dibutyrate binding in the hippocampus,” J. Neural. Transm., 108, 1065–1079 (2001).
G. M. Olsen, J. Scheel-Kruger, A. Moller, and L. H. Jensen, “Relation of spatial learning of rats in the Morris water maze task to the number of viable CA1 neurons following four-vessel occlusion,” Behav. Neurosci., 108, 681–690 (1994).
S. O'Mahony, T. Harkany, A. A. M. Rensink, I. Abraham, G. I. De Jong, J. L. Varga, M. Zarandi, B. Penke, C. Nyakas, P. G. Luiten, and B. E. Leonard, “β-Amyloid-induced cholinergic denervation correlates with enhanced nitric oxide synthase activity in rat cerebral cortex,: Reversal by NMDA receptor blockade,” Brain Res. Bull., 45, 405–411 (1998).
G. Paxinos and C. Watson, The Rat Brain in Stereotaxic Coordinates, Academic Press, Sydney (1982).
R. Schopke, D. P. Wolfe, H. P. Lipp, and M. C. Leisinger-Trigona, “Swimming navigation and structural variations of the infrapyramidal mossy fibres in the hippocampus of the mouse,” Hippocampus, 1, 315–328 (1991).
H. Schwegler and W. E. Crusio, “Correlations between radial-maze learning and structural variations of septum and hippocampus in rodents,” Behav. Brain Res., 67, 29–41 (1995).
H. Schwegler, G. G. Mueller, W. E. Crusio, L. Szemes, and L. Seress, “Hippocampal morphology and spatially related behavior in Long-Evans and CFY rats,” Hippocampus, 3, 1–7 (1993).
D. J. Selkoe, “Alzheimer's disease: genes, proteins, and therapy,” Physiol. Rev., 81, 741–766 (2001).
E. Shimizu, Y.-P. Tang, C. Rampon, and J. Tsien, “NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation,” Science, 290, 1170–1174 (2000).
L. R. Squire and S. Zola-Morgan, “Memory: brain systems and behavior,” Trends Neurosci., 11, 170–175 (1988).
M. Yu. Stepanichev, O. S. Mitrokhina, I. V. Viktorov, Yu. V. Moiseeva, N. A. Lazareva, M. V. Onufriev, and N. V. Gulyaeva, “β-Amyloid (25–35) induces impairment of working but not reference memory accompanied by neurodegeneration in rat brain,” in: Memory and Emotion, P. Calabrese and A. Neugebauer (eds.), World Sci., Singapore, New Jersey, London, Hong Kong (2002), pp. 445–452.
M. Yu. Stepanichev, Yu. V. Moiseeva, N. A. Lazareva, M. V. Onufriev, and N. V. Gulyaeva, “Single intracerebroventricular administration of amyloid beta (25–35) peptide induces impairment in short-term rather than long-term memory in rats,” Brain Res. Bull., 61, 197–205 (2003).
M. K. Sun and D. L. Alkon, “Impairment of hippocampal CA1 heterosynaptic transformation and spatial memory by beta-amyloid (25–35),” J. Neurophysiol., 87, 2441–2449 (2002).
O. S. Vinogradova, “Hippocampus as comparator: role of two input and two output systems of the hippocampus in selection and registration of information,” Hippocampus, 11, 578–598 (2001).
B. T. Volpe, H. P. Davis, A. Towle, and W. P. Dunlap, “Loss of hippocampal CA1 pyramidal neurons correlates with memory impairment in rats with ischemic or neurotoxic lesions,” Behav. Neurosci., 106, 457–464 (1992).
H. Wang, C. Chou, J. Liao, and C. Chen, “Dehydroevodiamine attenuates β-amyloid peptide-induced amnesia in mice,” Eur. J. Pharmacol., 413, 221–225 (2001).
G. M. Wittenberg and G. Z. Tsien, “An emerging molecular and cellular framework for memory processing by the hippocampus,” Trends Neurosci., 25, 501–505 (2002).
Y. Yamaguchi and S. Kawashima, “Effects of amyloid-β-(25–35) on passive avoidance, radial-arm maze learning, and choline acetyltransferase activity in the rat,” Eur. J. Pharmacol., 412, 265–272 (2001).
B. A. Yanker, L. K. Duffy, and D. A. Kirshner, “Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides,” Science, 250, 279–282 (1990).
Author information
Authors and Affiliations
Additional information
__________
Translated from Zhurnal Vysshei Nervnoi Deyatel'nosti imeni I. P. Pavlova, Vol. 54, No. 5, pp. 705–711, September–October, 2004.
Rights and permissions
About this article
Cite this article
Stepanichev, M.Y., Zdobnova, I.M., Zarubenko, I.I. et al. Studies of the Effects of Central Administration of β-Amyloid Peptide (25–35): Pathomorphological Changes in the Hippocampus and Impairment of Spatial Memory. Neurosci Behav Physiol 36, 101–106 (2006). https://doi.org/10.1007/s11055-005-0167-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11055-005-0167-1