Abstract
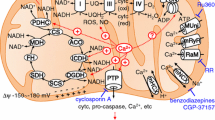
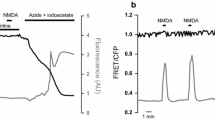
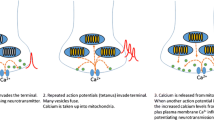
The malate–aspartate NADH shuttle (MAS) operates in neurons and other cells to translocate reducing equivalents from the cytosol to the mitochondrial matrix, thus allowing a continued flux through the glycolytic pathway and metabolism of extracellular lactate. Recent discoveries have taught us that MAS is regulated by fluctuations in cytosolic Ca2+ levels, and that this regulation is required to maintain a tight coupling between neuronal activity and mitochondrial respiration and oxidative phosphorylation. At cytosolic Ca2+ fluctuations below the threshold of the mitochondrial calcium uniporter, there is a positive correlation between Ca2+ and MAS activity; however, if cytosolic Ca2+ increases above the threshold, MAS activity is thought to be reduced by an intricate mechanism. The latter forces the neurons to partly rely on anaerobic glycolysis producing lactate that may be metabolized subsequently, by neurons or other cells. In this review, we will discuss the evidence for Ca2+-mediated regulation of MAS that have been uncovered over the last decade or so, together with the need for further verification, and examine the metabolic ramifications for neurons.
Similar content being viewed by others
References
Palmieri L, Pardo B, Lasorsa FM, del Arco A, Kobayashi K, Iijima M, Runswick MJ, Walker JE, Saheki T, Satrustegui J, Palmieri F (2001) Citrin and aralar1 are Ca(2+)-stimulated aspartate/glutamate transporters in mitochondria. EMBO J 20:5060–5069
del Arco A, Satrustegui J (1998) Molecular cloning of Aralar, a new member of the mitochondrial carrier superfamily that binds calcium and is present in human muscle and brain. J Biol Chem 273:23327–23334
del Arco A, Morcillo J, Martinez-Morales JR, Galian C, Martos V, Bovolenta P, Satrustegui J (2002) Expression of the aspartate/glutamate mitochondrial carriers aralar1 and citrin during development and in adult rat tissues. Eur J Biochem/FEBS 269:3313–3320
Ramos M, del Arco A, Pardo B, Martinez-Serrano A, Martinez-Morales JR, Kobayashi K, Yasuda T, Bogonez E, Bovolenta P, Saheki T, Satrustegui J (2003) Developmental changes in the Ca2+-regulated mitochondrial aspartate-glutamate carrier aralar1 in brain and prominent expression in the spinal cord. Brain Res Dev Brain Res 143:33–46
Contreras L, Urbieta A, Kobayashi K, Saheki T, Satrustegui J (2010) Low levels of citrin (SLC25A13) expression in adult mouse brain restricted to neuronal clusters. J Neurosci Res 88:1009–1016
Del Arco A, Agudo M, Satrustegui J (2000) Characterization of a second member of the subfamily of calcium-binding mitochondrial carriers expressed in human non-excitable tissues. Biochem J 345(Pt 3):725–732
Satrustegui J, Pardo B, Del Arco A (2007) Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol Rev 87:29–67
McKenna M, Dienel GA, Sonnewald U, Waagepetersen HS, Schousboe A (2012) Energy metabolism of the brain. In: Brady ST, Siegel GJ, Albers RW, Price DI (eds) Basic neurochmistry, 8th edn. Academic Press, Elsevier, Waltham, pp 200–231
Pardo B, Rodrigues TB, Contreras L, Garzon M, Llorente-Folch I, Kobayashi K, Saheki T, Cerdan S, Satrustegui J (2011) Brain glutamine synthesis requires neuronal-born aspartate as amino donor for glial glutamate formation. J Cereb Blood Flow Metab 31:90–101
Li B, Hertz L, Peng L (2012) Aralar mRNA and protein levels in neurons and astrocytes freshly isolated from young and adult mouse brain and in maturing cultured astrocytes. Neurochem Int 61:1325–1332
Genda EN, Jackson JG, Sheldon AL, Locke SF, Greco TM, O’Donnell JC, Spruce LA, Xiao R, Guo W, Putt M, Seeholzer S, Ischiropoulos H, Robinson MB (2011) Co-compartmentalization of the astroglial glutamate transporter, GLT-1, with glycolytic enzymes and mitochondria. J Neurosci 31:18275–18288
Contreras L, Satrustegui J (2009) Calcium signaling in brain mitochondria: interplay of malate aspartate NADH shuttle and calcium uniporter/mitochondrial dehydrogenase pathways. J Biol Chem 284:7091–7099
Contreras L, Gomez-Puertas P, Iijima M, Kobayashi K, Saheki T, Satrustegui J (2007) Ca2+ Activation kinetics of the two aspartate–glutamate mitochondrial carriers, aralar and citrin: role in the heart malate–aspartate NADH shuttle. J Biol Chem 282:7098–7106
Jalil MA, Begum L, Contreras L, Pardo B, Iijima M, Li MX, Ramos M, Marmol P, Horiuchi M, Shimotsu K, Nakagawa S, Okubo A, Sameshima M, Isashiki Y, Del Arco A, Kobayashi K, Satrustegui J, Saheki T (2005) Reduced N-acetylaspartate levels in mice lacking aralar, a brain- and muscle-type mitochondrial aspartate-glutamate carrier. J Biol Chem 280:31333–31339
Gellerich FN, Gizatullina Z, Arandarcikaite O, Jerzembek D, Vielhaber S, Seppet E, Striggow F (2009) Extramitochondrial Ca2+ in the nanomolar range regulates glutamate-dependent oxidative phosphorylation on demand. PLoS ONE 4:e8181
Gellerich FN, Gizatullina Z, Trumbekaite S, Korzeniewski B, Gaynutdinov T, Seppet E, Vielhaber S, Heinze HJ, Striggow F (2012) Cytosolic Ca2+ regulates the energization of isolated brain mitochondria by formation of pyruvate through the malate–aspartate shuttle. Biochem J 443:747–755
Gellerich FN, Gizatullina Z, Gainutdinov T, Muth K, Seppet E, Orynbayeva Z, Vielhaber S (2013) The control of brain mitochondrial energization by cytosolic calcium: the mitochondrial gas pedal. IUBMB Life 65:180–190
Mathiesen C, Caesar K, Thomsen K, Hoogland TM, Witgen BM, Brazhe A, Lauritzen M (2011) Activity-dependent increases in local oxygen consumption correlate with postsynaptic currents in the mouse cerebellum in vivo. J Neurosci 31:18327–18337
Llorente-Folch I, Rueda CB, Amigo I, del Arco A, Saheki T, Pardo B, Satrustegui J (2013) Calcium-regulation of mitochondrial respiration maintains ATP homeostasis and requires ARALAR/AGC1-malate aspartate shuttle in intact cortical neurons. J Neurosci 33(13957–13971):13971a
Pardo B, Contreras L, Serrano A, Ramos M, Kobayashi K, Iijima M, Saheki T, Satrustegui J (2006) Essential role of aralar in the transduction of small Ca2+ signals to neuronal mitochondria. J Biol Chem 281:1039–1047
Llorente-Folch I, Rueda CB, Pardo B, Szabadkai G, Duchen MR, Satrustegui J (2015) The regulation of neuronal mitochondrial metabolism by calcium. J Physiol (Lond). doi:10.1113/JP270254
Rueda CB, Llorente-Folch I, Amigo I, Contreras L, Gonzalez-Sanchez P, Martinez-Valero P, Juaristi I, Pardo B, del Arco A, Satrustegui J (2014) Ca(2+) regulation of mitochondrial function in neurons. Biochim Biophys Acta 1837:1617–1624
O’Donnell JM, Kudej RK, LaNoue KF, Vatner SF, Lewandowski ED (2004) Limited transfer of cytosolic NADH into mitochondria at high cardiac workload. Am J Physiol Heart Circ Physiol 286:H2237–H2242
O’Donnell JM, Doumen C, LaNoue KF, White LT, Yu X, Alpert NM, Lewandowski ED (1998) Dehydrogenase regulation of metabolite oxidation and efflux from mitochondria in intact hearts. Am J Physiol 274:H467–H476
McCormack JG, Denton RM (1979) The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J 180:533–544
Sluse FE, Goffart G, Liebecq C (1973) Mechanism of the exchanges catalysed by the oxoglutarate translocator of rat-heart mitochondria. Kinetics of the external-product inhibition. Eur J Biochem/FEBS 32:283–291
Porras OH, Loaiza A, Barros LF (2004) Glutamate mediates acute glucose transport inhibition in hippocampal neurons. J Neurosci 24:9669–9673
Bak LK, Walls AB, Schousboe A, Ring A, Sonnewald U, Waagepetersen HS (2009) Neuronal glucose but not lactate utilization is positively correlated with NMDA-induced neurotransmission and fluctuations in cytosolic Ca2+ levels. J Neurochem 109:87–93
Bak LK, Obel LF, Walls AB, Schousboe A, Faek SAA, Jajo FS, Waagepetersen HS (2012) Novel model of neuronal bioenergetics: postsynaptic utilization of glucose but not lactate correlates positively with Ca2+ signalling in cultured mouse glutamatergic neurons. Asn Neuro. doi:10.1042/AN20120004
Fox PT, Raichle ME, Mintun MA, Dence C (1988) Nonoxidative glucose consumption during focal physiologic neural activity. Science 241:462–464
Caesar K, Hashemi P, Douhou A, Bonvento G, Boutelle MG, Walls AB, Lauritzen M (2008) Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J Physiol 586:1337–1349
Bak LK, Schousboe A, Sonnewald U, Waagepetersen HS (2006) Glucose is necessary to maintain neurotransmitter homeostasis during synaptic activity in cultured glutamatergic neurons. J Cerebr Blood F Met 26:1285–1297
Pellerin L, Magistretti PJ (2012) Sweet sixteen for ANLS. J Cereb Blood Flow Metab 32:1152–1166
Hertz L, Gibbs ME, Dienel GA (2014) Fluxes of lactate into, from, and among gap junction-coupled astrocytes and their interaction with noradrenaline. Front Neurosci 8:261
Yang J, Ruchti E, Petit JM, Jourdain P, Grenningloh G, Allaman I, Magistretti PJ (2014) Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci USA 111:12228–12233
Hirrlinger J, Nave KA (2014) Adapting brain metabolism to myelination and long-range signal transduction. Glia 62:1749–1761
Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485:517–521
Jackson JG, O’Donnell JC, Takano H, Coulter DA, Robinson MB (2014) Neuronal activity and glutamate uptake decrease mitochondrial mobility in astrocytes and position mitochondria near glutamate transporters. J Neurosci 34:1613–1624
Johnson DT, Harris RA, French S, Blair PV, You J, Bemis KG, Wang M, Balaban RS (2007) Tissue heterogeneity of the mammalian mitochondrial proteome. Am J Physiol Cell Physiol 292:C689–C697
Johnson DT, Harris RA, Blair PV, Balaban RS (2007) Functional consequences of mitochondrial proteome heterogeneity. Am J Physiol Cell Physiol 292:C698–C707
Waagepetersen HS, Hansen GH, Fenger K, Lindsay JG, Gibson G, Schousboe A (2006) Cellular mitochondrial heterogeneity in cultured astrocytes as demonstrated by immunogold labeling of alpha-ketoglutarate dehydrogenase. Glia 53:225–231
Waagepetersen HS, Sonnewald U, Schousboe A (2003) Compartmentation of glutamine, glutamate, and GABA metabolism in neurons and astrocytes: functional implications. Neuroscientist 9:398–403
Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT (2009) Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61:541–555
Dienel GA, McKenna MC (2014) A dogma-breaking concept: glutamate oxidation in astrocytes is the source of lactate during aerobic glycolysis in resting subjects. J Neurochem 131:395–398
Dienel GA (2014) Lactate shuttling and lactate use as fuel after traumatic brain injury: metabolic considerations. J Cereb Blood Flow Metab 34:1736–1748
Dienel GA (2012) Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab 32:1107–1138
Dienel GA, Cruz NF (2009) Exchange-mediated dilution of brain lactate specific activity: implications for the origin of glutamate dilution and the contributions of glutamine dilution and other pathways. J Neurochem 109(Suppl 1):30–37
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91:10625–10629
Ivanov AI, Malkov AE, Waseem T, Mukhtarov M, Buldakova S, Gubkina O, Zilberter M, Zilberter Y (2014) Glycolysis and oxidative phosphorylation in neurons and astrocytes during network activity in hippocampal slices. J Cereb Blood Flow Metab 34:397–407
Patel AB, Lai JC, Chowdhury GM, Hyder F, Rothman DL, Shulman RG, Behar KL (2014) Direct evidence for activity-dependent glucose phosphorylation in neurons with implications for the astrocyte-to-neuron lactate shuttle. Proc Natl Acad Sci USA 111:5385–5390
Lewandowski ED, O’Donnell JM, Scholz TD, Sorokina N, Buttrick PM (2007) Recruitment of NADH shuttling in pressure-overloaded and hypertrophic rat hearts. Am J Physiol Cell Physiol 292:C1880–C1886
Josan S, Park JM, Hurd R, Yen YF, Pfefferbaum A, Spielman D, Mayer D (2013) In vivo investigation of cardiac metabolism in the rat using MRS of hyperpolarized [1-13C] and [2-13C]pyruvate. NMR Biomed 26:1680–1687
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: In honor of Dr. Gerald Dienel.
Rights and permissions
About this article
Cite this article
Satrústegui, J., Bak, L.K. Fluctuations in Cytosolic Calcium Regulate the Neuronal Malate–Aspartate NADH Shuttle: Implications for Neuronal Energy Metabolism. Neurochem Res 40, 2425–2430 (2015). https://doi.org/10.1007/s11064-015-1652-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1652-8