Abstract
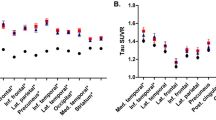
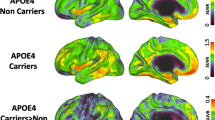
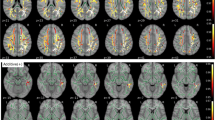
Many studies have investigated APOE-related differences in cerebral structure, blood flow, metabolism, and activation in an attempt to detect early brain changes in subjects at risk for Alzheimer’s disease (AD). Structural magnetic resonance imaging studies have produced conflicting results, with some failing to detect APOE-related differences and others suggesting that ε4 carriers have more pronounced atrophy, particularly at medial temporal structures. All functional imaging studies done during rest in middle-aged and elderly subjects have found decreased cerebral metabolism for ε4 carriers (mostly in areas that usually are affected by AD), and some have reported faster cerebral metabolic reductions over time. Areas with decreased resting cerebral perfusion and metabolism, in addition to other areas with increased perfusion, have been reported in young ε4 carriers. Imaging studies done during the performance of various cognitive tasks in middle-aged and elderly subjects, and a single study in young subjects, have produced mixed results with regionally nonspecific increased, decreased, or nondifferential APOE-related activations depending on the cognitive task used. APOE-related findings in imaging studies of nondemented subjects may be the result of incipient AD pathologic changes or of genetic heterogeneity in brain structure and function.
Similar content being viewed by others
References and Recommended Reading
Corder EH, Saunders AM, Strittmatter WJ, et al.: Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261:921–923.
Saunders AM, Strittmatter WJ, Schmechel D, et al.: Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993, 43:1467–1472.
Alzheimer’s Disease Collaborative Group: Apolipoprotein E genotype and Alzheimer’s disease. Lancet 1993, 342:737–738.
Corder EH, Saunders AM, Risch NJ, et al.: Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 1994, 7:180–184.
Scarmeas N, Brandt J, Albert M, et al.: Association between the APOE genotype and psychopathologic symptoms in Alzheimer’s disease. Neurology 2002, 58:1182–1188.
Jack Jr CR, Petersen RC, Xu YC, et al.: Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer’s disease. Ann Neurol 1998, 43:303–310.
Killiany RJ, Hyman BT, Gomez-Isla T, et al.: MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology 2002, 58:1188–1196.
Moffat SD, Szekely CA, Zonderman AB, et al.: Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology 2000, 55:134–136.
Alexander GE, Chen K, Reiman EM, et al.: APOE e4 dose effect on gray matter atrophy in cognitively normal adults. Society for Neuroscience Conference 2003.
Soininen H, Partanen K, Pitkanen A, et al.: Decreased hippocampal volume asymmetry on MRIs in nondemented elderly subjects carrying the apolipoprotein E epsilon 4 allele. Neurology 1995, 45:391–392.
den HeijerT, Oudkerk M, Launer LJ, et al.: Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology 2002, 59:746–748. The largest structural MRI study (including 1077 nondemented subjects) examining associations between APOE genotype and cerebral atrophy.
Fleisher A, Grundman M, Jack Jr CR, et al.: Sex, apolipoprotein Erepsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol 2005, 62:953–957.
Plassman BL, Welsh-Bohmer KA, Bigler ED, et al.: Apolipoprotein E epsilon 4 allele and hippocampal volume in twins with normal cognition. Neurology 1997, 48:985–989.
Small GW, Mazziotta JC, Collins MT, et al.: Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA 1995, 273:942–947.
Small GW, Ercoli LM, Silverman DH, et al.: Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A 2000, 97:6037–6042.
Reiman EM, Caselli RJ, Yun LS, et al.: Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med 1996, 334:752–758.
Reiman EM, Chen K, Alexander GE, et al.: Correlations between apolipoprotein E epsilon4 gene dose and brainimaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A 2005, 102:8299–8302. A PET study including 160 cognitively normal middle-aged and elderly subjects. It showed that ⇘4 allele dose was correlated with lower cerebral metabolism during rest in brain regions that usually are affected by AD.
de LeonMJ, Convit A, Wolf OT, et al.: Prediction of cognitive decline in normal elderly subjects with 2-[(18)F].uoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET). Proc Natl Acad Sci U S A 2001, 98:10966–10971.
Reiman EM, Chen K, Alexander GE, et al.: Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A 2004, 101:284–289. This study reported low rates of cerebral metabolism during rest for cognitively healthy ⇘4 carriers aged 20 to 39 years. Therefore, functional brain changes for carriers of a common susceptibility gene for AD can be detected already in young adulthood, several decades before the possible onset of dementia.
Scarmeas N, Habeck CG, Stern Y, Anderson KE: APOE genotype and cerebral blood flow in healthy young individuals. JAMA 2003, 290:1581–1582. The first report showing that APOE-related differences in PETassessed cerebral perfusion exist, even at college age.
Burggren AC, Small GW, Sabb FW, Bookheimer SY: Speci.city of brain activation patterns in people at genetic risk for Alzheimer disease. Am J Geriatr Psychiatry 2002, 10:44–51.
Smith CD, Andersen AH, Kryscio RJ, et al.: Altered brain activation in cognitively intact individuals at high risk for Alzheimer’s disease. Neurology 1999, 53:1391–1396.
Smith CD, Andersen AH, Kryscio RJ, et al.: Women at risk for AD show increased parietal activation during a fluency task. Neurology 2002, 58:1197–1202.
Bondi MW, Houston WS, Eyler LT, Brown GG: fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 2005, 64:501–508.
Scarmeas N, Habeck C, Anderson KE, et al.: Altered PET Functional Brain Responses in Cognitively Intact Elderly Persons at Risk for Alzheimer Disease (Carriers of the silon4 Allele). Am J Geriatr Psychiatry 2004, 12:596–605. This study reported APOE-related differences in PET activation of 32 nondemented elderly during performance of a nonverbal memory task. The fact that task difficulty was experimentally equated among participants indicated that differences in activation are a result of APOE-related altered memory processing and not of differential effort during task performance.
Petrella JR, Lustig C, Bucher LA, et al.: Prefrontal activation patterns in subjects at risk for Alzheimer disease. Am J Geriatr Psychiatry 2002, 10:112–113.
Bookheimer SY, Strojwas MH, Cohen MS, et al.: Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med 2000, 343:450–456. One of the earliest fMRI studies examining associations between APOE status and brain activation. Increased magnitude and extend of fMRI activation was noted for ⇘4 carriers during performance of a memory task, which was interpreted as evidence of compensation.
Dickerson BC, Salat DH, Greve DN, et al.: Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 2005, 65:404–411.
Scarmeas N, Habeck CG, Hilton J, et al.: APOE related alterations in cerebral activation even at college age. J Neurol Neurosurg Psychiatry 2005, 76:1440–1444. This is the only study that has examined differences in cerebral activation for cognitively healthy young subjects of different APOE genotypes. With use of PET during performance of a nonverbal memory task, the authors reported signi.cant APOE-related differences in brain activation, even at college age.
Lehtovirta M, Laakso MP, Soininen H, et al.: Volumes of hippocampus, amygdala and frontal lobe in Alzheimer patients with different apolipoprotein E genotypes. Neuroscience 1995, 67:65–72.
Drzezga A, Riemenschneider M, Strassner B, et al.: Cerebral glucose metabolism in patients with AD and different APOE genotypes. Neurology 2005, 64:102–107.
Corder EH, Jelic V, Basun H, et al.: No difference in cerebral glucose metabolism in patients with Alzheimer disease and differing apolipoprotein E genotypes. Arch Neurol 1997, 54:273–277.
Scarmeas N, Anderson KE, Hilton J, et al.: APOE-dependent PET patterns of brain activation in Alzheimer disease. Neurology 2004, 63:913–915.
Rose A, Addington A, Clasen LS, et al.: The relationship between apolipoprotein E, cognition, and hippocampal development in healthy pediatric subjects. Society for Neuroscience Conference. New Orleans, LA; November 8–12, 2003.
Reiman EM, Caselli RJ, Chen K, et al.: Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci U S A 2001, 98:3334–3339.
Rypma B, Prabhakaran V, Desmond JE, et al.: Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage 1999, 9:216–226.
Grady CL, Horwitz B, Pietrini P, et al.: The effect of task difficulty on cerebral blood flow during perceptual matching of faces. Hum Brain Mapp 1996, 4:227–239.
Srinivasan SR, Ehnholm C, Elkasabany A, Berenson GS: Apolipoprotein E polymorphism modulates the association between obesity and dyslipidemias during young adulthood: The Bogalusa Heart Study. Metabolism 2001, 50:696–702.
Katsuya T, Baba S, Ishikawa K, et al.: Epsilon 4 allele of apolipoprotein E gene associates with lower blood pressure in young Japanese subjects: the Suita Study. J Hypertens 2002, 20:2017–2021.
Hixson JE: Apolipoprotein E polymorphisms affect atherosclerosis in young males. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb 1991, 11:1237–1244.
van Bockxmeer FM, Mamotte CD: Apolipoprotein epsilon 4 homozygosity in young men with coronary heart disease. Lancet 1992, 340:879–880.
Brscic E, Bergerone S, Gagnor A, et al.: Acute myocardial infarction in young adults: prognostic role of angiotensin-converting enzyme, angiotensin II type I receptor, apolipoprotein E, endothelial constitutive nitric oxide synthase, and glycoprotein IIIa genetic polymorphisms at medium-term follow-up. Am Heart J 2000, 139:979–984.
Ferguson SC, Deary IJ, Evans JC, et al.: Apolipoprotein-e in.uences aspects of intellectual ability in type 1 diabetes. Diabetes 2003, 52:145–148.
Rask-Nissila L, Jokinen E, Viikari J, et al.: Impact of dietary intervention, sex, and apolipoprotein E phenotype on tracking of serum lipids and apolipoproteins in 1-to 5-year-old children: the Special Turku Coronary Risk Factor Intervention Project (STRIP). Arterioscler Thromb Vasc Biol 2002, 22:492–498.
Tammi A, Ronnemaa T, Rask-Nissila L, et al.: Apolipoprotein E phenotype regulates cholesterol absorption in healthy 13-month-old children--The STRIP Study. Pediatr Res 2001, 50:688–691.
Zhou Y, Elkins PD, Howell LA, et al.: Apolipoprotein-E de.ciency results in an altered stress responsiveness in addition to an impaired spatial memory in young mice. Brain Res 1998, 788:151–159.
Valastro B, Ghribi O, Poirier J, et al.: AMPA receptor regulation and LTP in the hippocampus of young and aged apolipoprotein E-de.cient mice. Neurobiol Aging 2001, 22:9–15.
Selkoe DJ: Alzheimer’s disease is a synaptic failure. Science 2002, 298:789–791.
Richey PL, Siedlak SL, Smith MA, Perry G: Apolipoprotein E interaction with the neuro.brillary tangles and senile plaques in Alzheimer disease: implications for disease pathogenesis. Biochem Biophys Res Commun 1995, 208:657–663.
Polvikoski T, Sulkava R, Haltia M, et al.: Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med 1995, 333:1242–1247.
Morishima-Kawashima M, Oshima N, Ogata H, et al.: Effect of apolipoprotein E allele epsilon4 on the initial phase of amyloid beta-protein accumulation in the human brain. Am J Pathol 2000, 157:2093–2099.
Ghebremedhin E, Schultz C, Braak E, Braak H: High frequency of apolipoprotein E epsilon4 allele in young individuals with very mild Alzheimer’s disease-related neuro.brillary changes. Exp Neurol 1998, 153:152–155.
Lehtovirta M, Laakso MP, Soininen H, et al.: Volumes of hippocampus, amygdala and frontal lobe in Alzheimer patients with different apolipoprotein E genotypes. Neuroscience 1995, 67:65–72.
Lehtovirta M, Soininen H, Helisalmi S, et al.: Clinical and neuropsychological characteristics in familial and sporadic Alzheimer’s disease: relation to apolipoprotein E polymorphism. Neurology 1996, 46:413–419.
Rypma B, D’Esposito M: Age-related changes in brainbehaviour relationships: evidence from event-related functional MRI studies. Eur J Cogn Psychol 2001, 13:235–256.
Madden DJ, Turkington TG, Provenzale JM, et al.: Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Mapp 1999, 7:115–135.
Logan JM, Sanders AL, Snyder AZ, et al.: Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron 2002, 33:827–840.
Becker JT, Mintun MA, Aleva K, et al.: Compensatory reallocation of brain resources supporting verbal episodic memory in Alzheimer’s disease. Neurology 1996, 46:692–700.
Meyer MR, Tschanz JT, Norton MC, et al.: APOE genotype predicts when—not whether—one is predisposed to develop Alzheimer’s disease. Nat Genet 1998, 19:321–322.
Teter B, Xu PT, Gilbert JR, et al.: Human apolipoprotein E isoform-speci.c differences in neuronal sprouting in organotypic hippocampal culture. J Neurochem 1999, 73:2613–2616.
Buttini M, Orth M, Bellosta S, et al.: Expression of human apolipoprotein E3 or E4 in the brains of Apoe-/-mice: isoform-speci.c effects on neurodegeneration. J Neurosci 1999, 19:4867–4880.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scarmeas, N., Stern, Y. Imaging studies and APOE genotype in persons at risk for Alzheimer’s disease. Curr Psychiatry Rep 8, 11–17 (2006). https://doi.org/10.1007/s11920-006-0076-1
Issue Date:
DOI: https://doi.org/10.1007/s11920-006-0076-1