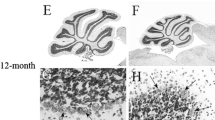
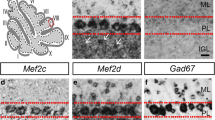
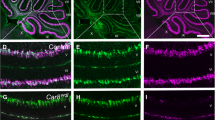
Abstract
The Lurcher mutant mouse is characterized by its ataxic gait and loss of cerebellar Purkinje cells and their afferents, granule cells and olivary neurons, during the first weeks of postnatal development. For the 50 years since its discovery, the heterozygous Lurcher mutant has served as an important model system for studying neuron–target interactions in the developing cerebellum and cerebellar function. The identification of the Lurcher (Lc) gene over 10 years ago as a gain-of-function mutation in the δ2 glutamate receptor (GluRδ2) led to extensive studies of cell death mechanisms in the Lc/+ cerebellum. The advantage of this model system is that GluRδ2+ receptors and GluRδ2Lc channels are expressed predominantly in Purkinje cells, making it possible to study the effects of a well-characterized leak current in a well-defined cell type during a critical phase of neuronal development. Yet there is still controversy surrounding the mechanisms of neuronal death in Lc/+ Purkinje cells with competing hypotheses for necrotic, apoptotic, and autophagic cell death pathways as a consequence of the excitotoxic stress caused by the GluRδ2Lc leak current. The goal of this review is to summarize recent studies that critically test the role of various cell death pathways in Lc/+ Purkinje cell degeneration with respect to evidence for the molecular heterogeneity of Purkinje cells. We propose that the expression of putative survival factors, such as heat shock proteins, in a subset of cerebellar Purkinje cells may affect cell death pathways and account for the pattern and diverse mechanisms of Lc/+ Purkinje degeneration.



Similar content being viewed by others
References
Vogel MW, Caston J, Yuzaki M, Mariani J. The Lurcher mouse: fresh insights from an old mutant. Brain Res. 2007;1140:4–18.
Armstrong CL, Vogel MW, Hawkes R. Development of Hsp25 expression compartments is not constrained by Purkinje cell defects in the Lurcher mouse mutant. J Comp Neurol. 2005;491(1):69–78.
Martin LA, Goldowitz D, Mittleman G. Sustained attention in the mouse: a study of the relationship with the cerebellum. Behav Neurosci. 2006;120(2):477–81.
Martin LA, Escher T, Goldowitz D, Mittleman G. A relationship between cerebellar Purkinje cells and spatial working memory demonstrated in a lurcher/chimera mouse model system. Genes Brain Behav. 2004;3(3):158–66.
Martin LA, Goldowitz D, Mittleman G. Repetitive behavior and increased activity in mice with Purkinje cell loss: a model for understanding the role of cerebellar pathology in autism. Eur J Neurosci. 2010;31(3):544–5.
Caston J, Chianale C, Delhaye-Bouchaud N, Mariani J. Role of the cerebellum in exploration behavior. Brain Res. 1998;808(2):232–7.
Caston J, Devulder B, Jouen F, Lalonde R, Delhaye-Bouchaud N, Mariani J. Role of an enriched environment on the restoration of behavioral deficits in Lurcher mutant mice. Dev Psychobiol. 1999;35(4):291–303.
Caston J, Lalonde R, Delhaye-Bouchaud N, Mariani J. The cerebellum and postural sensorimotor learning in mice and rats. Behav Brain Res. 1998;95(1):17–22.
Caston J, Vasseur F, Delhaye-Bouchaud N, Mariani J. Delayed spontaneous alternation in intact and cerebellectomized control and lurcher mutant mice: differential role of cerebellar cortex and deep cerebellar nuclei. Behav Neurosci. 1997;111(1):214–8.
Mittleman G, Goldowitz D, Heck DH, Blaha CD. Cerebellar modulation of frontal cortex dopamine efflux in mice: relevance to autism and schizophrenia. Synapse. 2008;62(7):544–50.
Monfort V, Chapillon P, Mellier D, Lalonde R, Caston J. Timed active avoidance learning in lurcher mutant mice. Behav Brain Res. 1998;91(1–2):165–72.
Vogel MW. Cell death, Bcl-2, Bax, and the cerebellum. Cerebellum. 2002;1:277–88.
Dusart I, Guenet JL, Sotelo C. Purkinje cell death: differences between developmental cell death and neurodegenerative death in mutant mice. Cerebellum. 2006;5(2):163–73.
Yue Z, Horton A, Bravin M, DeJager PL, Selimi F, Heintz N. A novel protein complex linking the delta 2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice. Neuron. 2002;35(5):921–33.
Nishiyama J, Matsuda K, Kakegawa W, Yamada N, Motohashi J, Mizushima N, et al. Reevaluation of neurodegeneration in lurcher mice: constitutive ion fluxes cause cell death with, not by, autophagy. J Neurosci. 2010;30(6):2177–87.
Zuo J, De Jager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N. Neurodegeneration in Lurcher mice caused by mutation in δ2 glutamate receptor. Nature. 1997;388:769–73.
Heintz N, Zoghbi HY. Insights from mouse models into the molecular basis of neurodegeneration. Annu Rev Physiol. 2000;62:779–802.
Cheng SSW, Heintz N. Massive loss of mid- and hindbrain neurons during embryonic development of homozygous Lurcher mice. J Neurosci. 1997;17:2400–7.
Caddy KWT, Biscoe TJ. Structural and quantitative studies on the normal C3H and Lurcher mutant mouse. Phil Trans Roy Soc Lond B. 1979;287:167–201.
Duffin CA, McFarland R, Sarna JR, Vogel MW, Armstrong CL. Heat shock protein 25 expression and preferential Purkinje cell survival in the Lurcher mutant mouse cerebellum. J Comp Neurol. 2010;518:1892–907.
Wetts R, Herrup K. Interaction of granule, Purkinje and inferior olivary neurons in lurcher chimaeric mice. I. Qualitative studies. J Embryol Exp Morphol. 1982;68:87–98.
Wetts R, Herrup K. Interaction of granule, Purkinje and inferior olivary neurons in lurcher chimeric mice. II. Granule cell death. Brain Res. 1982;250:358–63.
Araki K, Meguro H, Kushiya E, Takayama C, Inoue Y, Mishina M. Selective expression of the glutamate receptor channel d2 subunit in cerebellar Purkinje cells. Biochem Biophys Res Comm. 1993;197:1267–76.
Lomeli H, Sprengel R, Lauris DJ, Kohr G, Herb A, Seeburg PH, et al. The rat delta-1 and delta-2 subunits extend the excitatory amino acid receptor family. FEBS Lett. 1993;315:318–22.
Takayama C, Nakagawa S, Watanabe M, Mishina M, Inoue Y. Developmental changes in expression and distribution of the glutamate receptor channel d2 subunit according to the Purkinje cell maturation. Devel Brain Res. 1996;92:147–55.
Dumesnil-Bousez N, Sotelo C. Early development of the Lurcher cerebellum: Purkinje cell alterations and impairment of synaptogenesis. J Neurocytol. 1992;21:506–29.
Norman DJ, Feng L, Cheng SS, Gubbay J, Chan E, Heintz N. The lurcher gene induces apoptotic death in cerebellar Purkinje cells. Development. 1995;121(4):1183–93.
Lu W, Tsirka SE. Patial rescue of neural apoptosis in the Lurcher mutant mouse through elimination of tissue plasminogen activator. Development. 2002;129:2043–50.
Selimi F, Vogel MW, Mariani J. Bax inactivation in Lurcher mutants rescues cerebellar granule cells but not Purkinje cells or inferior olivary neurons. J Neurosci. 2000;20(14):5339–45.
Wullner U, Weller M, Schulz J, Krajewski S, Reed J, Klockgether T. Bcl-2, Bax and Bcl-x expression in neuronal apoptosis: a study of mutant weaver and lurcher mice. Acta Neuropathol (Berl). 1998;96:233–8.
Wullner U, Loschmann PA, Weller M, Klockgether T. Apoptotic cell death in the cerebellum of mutant weaver and lurcher mice. Neurosci Lett. 1995;200(2):109–12.
Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, et al. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26(31):8057–68.
Yue Z. Autophagy in lurcher mice: indicted but yet to be acquitted for the death of Purkinje cells. Autophagy. 2010;6(4):1–2.
Sarna JR, Hawkes R. Patterned Purkinje cell death in the cerebellum. Prog Neurobiol. 2003;70(6):473–507.
Arpino C, D’Argenzio L, Ticconi C, Di Paolo A, Stellin V, Lopez L, et al. Brain damage in preterm infants: etiological pathways. Ann Ist Super Sanita. 2005;41(2):229–37.
Welsh JP, Yuen G, Placantonakis DG, Vu TQ, Haiss F, O’Hearn E, et al. Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol. 2002;89:331–59.
Brorson JR, Manzolillo PA, Gibbons SJ, Miller RJ. AMPA receptor desensitization predicts the selective vulnerability of cerebellar Purkinje cells to excitotoxicity. J Neurosci. 1995;15:4515–24.
Kitao Y, Hashimoto K, Matsuyama T, Iso H, Tamatani T, Hori O, et al. ORP150/HSP12A regulates Purkinje cell survival: a role for endoplasmic reticulum stress in cerebellar development. J Neurosci. 2004;24(6):1486–96.
Yuan HB, Huang Y, Zheng S, Zuo Z. Hypothermic preconditioning increases survival of Purkinje neurons in rat cerebellar slices after an in vitro simulated ischemia. Anesthesiology. 2004;100(2):331–7.
Sargent MA, Poskitt KJ, Roland EH, Hill A, Hendson G. Cerebellar vermian atrophy after neonatal hypoxic–ischemic encephalopathy. AJNR Am J Neuroradiol. 2004;25(6):1008–15.
Leiner HC, Leiner AL, Dow RS. The human cerebro-cerebellar system: its computing, cognitive, and language skills. Behav Brain Res. 1991;44:113–28.
Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129(Pt 2):290–2.
Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–78.
Wiser AK, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Dysfunctional cortico-cerebellar circuits cause ‘cognitive dysmetria’ in schizophrenia. Neuroreport. 1998;9(8):1895–9.
Doughty ML, De Jager PL, Korsmeyer SJ, Heintz N. Neurodegeneration in Lurcher mice occurs via multiple cell death pathways. J Neurosci. 2000;20(10):3687–94.
Selimi F, Doughty M, Delhaye-Bouchaud N, Mariani J. Target-related and intrinsic neuronal death in Lurcher mutant mice are both mediated by caspase-3 activation. J Neurosci. 2000;20:992–1000.
Zanjani HS, Rondi-Reig L, Vogel MW, Martinou JC, Delhaye-Bouchaud N, Mariani J. Overexpression of a Hu-Bcl-2 gene in Lurcher mutant mice delays Purkinje cell death. C R Acad Sci III. 1998;321:633–40.
Zanjani HS, Vogel MW, Martinou JC, Delhaye-Bouchaud N, Mariani J. Postnatal expression of Hu-bcl-2 gene in Lurcher mutant mice fails to rescue Purkinje cells but protects inferior olivary neurons from target-related cell death. J Neurosci. 1998;18(1):319–27.
Nishiyama J, Yuzaki M. Excitotoxicity and autophagy: lurcher may not be a model of “autophagic cell death”. Autophagy. 2010;6(4):568–70.
Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72(11):8586–96.
Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402(6762):672–6.
Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin–phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2(4):330–5.
Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–39.
Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4(2):151–75.
He C, Levine B. The Beclin 1 interactome. Curr Opin Cell Biol. 2010;22(2):140–9.
Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene. 2008;27 Suppl 1:S137–48.
Cecconi F, Levine B. The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell. 2008;15(3):344–57.
Carling D. The role of the AMP-activated protein kinase in the regulation of energy homeostasis. Novartis Found Symp. 2007;286:72–81. discussion 81–5, 162–3, 196–203.
Meijer AJ, Codogno P. AMP-activated protein kinase and autophagy. Autophagy. 2007;3(3):238–40.
Yuzaki M, Nishiyama J. A response to Dr. Yue’s commentary. Autophagy. 2010;6(4) [Epub ahead of print].
Takayama C, Nakagawa S, Watanabe M, Mishina M, Inoue Y. Developmental changes in expression and distribution of the glutamate receptor channel delta 2 subunit according to the Purkinje cell maturation. Brain Res Dev Brain Res. 1996;92(2):147–55.
Wilkinson JM, Pollard I. Immunohistochemical localisation of the 25 kDa heat shock protein in unstressed rats: possible functional implications. Anat Rec. 1993;237(4):453–7.
Plumier JC, Hopkins DA, Robertson HA, Currie RW. Constitutive expression of the 27-kDa heat shock protein (Hsp27) in sensory and motor neurons of the rat nervous system. J Comp Neurol. 1997;384(3):409–28.
Kalesnykas G, Niittykoski M, Rantala J, Miettinen R, Salminen A, Kaarniranta K, et al. The expression of heat shock protein 27 in retinal ganglion and glial cells in a rat glaucoma model. Neuroscience. 2007;150(3):692–704.
Armstrong CL, Krueger-Naug AM, Currie RW, Hawkes R. Constitutive expression of the 25-kDa heat shock protein Hsp25 reveals novel parasaggital bands of Purkinje cells in the adult mouse cerebellar cortex. J Comp Neurol. 2000;416:383–97.
Armstrong CL, Krueger-Naug AM, Currie RW, Hawkes R. Expression of heat-shock protein Hsp25 in mouse Purkinje cells during development reveals novel features of cerebellar compartmentation. J Comp Neurol. 2001;429(1):7–21.
Wagstaff MJ, Collaco-Moraes Y, Smith J, de Belleroche JS, Coffin RS, Latchman DS. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J Biol Chem. 1999;274(8):5061–9.
Quigney DJ, Gorman AM, Samali A. Heat shock protects PC12 cells against MPP+ toxicity. Brain Res. 2003;993(1–2):133–9.
Akbar MT, Lundberg AM, Liu K, Vidyadaran S, Wells KE, Dolatshad H, et al. The neuroprotective effects of heat shock protein 27 overexpression in transgenic animals against kainate-induced seizures and hippocampal cell death. J Biol Chem. 2003;278(22):19956–65.
Maatkamp A, Vlug A, Haasdijk E, Troost D, French PJ, Jaarsma D. Decrease of Hsp25 protein expression precedes degeneration of motoneurons in ALS-SOD1 mice. Eur J Neurosci. 2004;20(1):14–28.
Tolbert DL, Ewald M, Gutting J, La Regina MC. Spatial and temporal pattern of Purkinje cell degeneration in shaker mutant rats with hereditary cerebellar ataxia. J Comp Neurol. 1995;355(4):490–507.
Duchala CS, Shick HE, Garcia J, Deweese DM, Sun X, Stewart VJ, et al. The toppler mouse: a novel mutant exhibiting loss of Purkinje cells. J Comp Neurol. 2004;476(2):113–29.
Rossi D, Cozzio A, Flechsig E, Klein MA, Rulicke T, Aguzzi A, et al. Onset of ataxia and Purkinje cell loss in PrP null mice inversely correlated with Dpl level in brain. Embo J. 2001;20(4):694–702.
Sarna JR, Larouche M, Marzban H, Sillitoe RV, Rancourt DE, Hawkes R. Patterned Purkinje cell degeneration in mouse models of Niemann–Pick type C disease. J Comp Neurol. 2003;456(3):279–91.
Sarna J, Miranda SR, Schuchman EH, Hawkes R. Patterned cerebellar Purkinje cell death in a transgenic mouse model of Niemann Pick type A/B disease. Eur J Neurosci. 2001;13(10):1873–80.
Goldbaum O, Riedel M, Stahnke T, Richter-Landsberg C. The small heat shock protein HSP25 protects astrocytes against stress induced by proteasomal inhibition. Glia. 2009;57(14):1566–77.
Santoro MG. Heat shock factors and the control of the stress response. Biochem Pharmacol. 2000;59(1):55–63.
Franklin TB, Krueger-Naug AM, Clarke DB, Arrigo AP, Currie RW. The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system. Int J Hyperthermia. 2005;21(5):379–92.
Preville X, Gaestel M, Arrigo AP. Phosphorylation is not essential for protection of L929 cells by Hsp25 against H2O2-mediated disruption actin cytoskeleton, a protection which appears related to the redox change mediated by Hsp25. Cell Stress Chaperones. 1998;3(3):177–87.
Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, et al. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem. 2003;278(30):27828–35.
Schepers H, Geugien M, van der Toorn M, Bryantsev AL, Kampinga HH, Eggen BJ, et al. HSP27 protects AML cells against VP-16-induced apoptosis through modulation of p38 and c-Jun. Exp Hematol. 2005;33(6):660–70.
Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. Faseb J. 1999;13(14):2061–70.
Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2(9):645–52.
Samali A, Robertson JD, Peterson E, Manero F, van Zeijl L, Paul C, et al. Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 2001;6(1):49–58.
Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol. 2002;22(3):816–34.
Chen S, Brown IR. Neuronal expression of constitutive heat shock proteins: implications for neurodegenerative diseases. Cell Stress Chaperones. 2007;12(1):51–8.
Stetler RA, Gao Y, Signore AP, Cao G, Chen J. HSP27: mechanisms of cellular protection against neuronal injury. Curr Mol Med. 2009;9(7):863–72.
Yuzaki M. The delta2 glutamate receptor: 10 years later. Neurosci Res. 2003;46(1):11–22.
Golden WC, Brambrink AM, Traystman RJ, Shaffner DH, Martin LJ. Nitration of the striatal Na, K-ATPase alpha3 isoform occurs in normal brain development but is not increased during hypoxia–ischemia in newborn piglets. Neurochem Res. 2003;28(12):1883–9.
Reifenberger MS, Arnett KL, Gatto C, Milanick MA. The reactive nitrogen species peroxynitrite is a potent inhibitor of renal Na–K-ATPase activity. Am J Physiol Renal Physiol. 2008;295(4):F1191–8.
Lin D, Takemoto DJ. Oxidative activation of protein kinase Cgamma through the C1 domain. Effects on gap junctions. J Biol Chem. 2005;280(14):13682–93.
Knapp LT, Kanterewicz BI, Hayes EL, Klann E. Peroxynitrite-induced tyrosine nitration and inhibition of protein kinase C. Biochem Biophys Res Commun. 2001;286(4):764–70.
Chakraborti T, Das S, Chakraborti S. Proteolytic activation of protein kinase Calpha by peroxynitrite in stimulating cytosolic phospholipase A2 in pulmonary endothelium: involvement of a pertussis toxin sensitive protein. Biochemistry. 2005;44(13):5246–57.
Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev. 2008;88(4):1341–78.
Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40(6):928–39. Epub 2005 Nov 21.
Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–7.
Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29(3):629–43.
Becker EB, Howell J, Kodama Y, Barker PA, Bonni A. Characterization of the c-Jun N-terminal kinase–BimEL signaling pathway in neuronal apoptosis. J Neurosci. 2004;24(40):8762–70.
McFarland RJ, Vogel MW. Increased a3 subunit expression but decreased Na+/K+ ATPase activity in Lurcher Purkinje cells. Program Number 354.8. Neuroscience Meeting Planner. Society for Neuroscience, 2008. Online. 2008.
Zanjani HS, Gautheron V, Vogel MW, Mariani J. Protein Kinase C (PKC) activity is involved both in developmental and pathological Purkinje cell death in Lurcher mutant mice. Program Number 509.10. 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2009. Online. 2009.
Zanjani HS, Repici M, Levenes C, Vogel MW, Mariani J. Both c-Jun N-terminal (JNK) and p38 MAP kinases pathways are involved in Lurcher Purkinje cells death. Program Number 355.1. Neuroscience Meeting Planner. Society for Neuroscience, 2008. Online. 2008.
Zanjani HS, Gautheron V, Dusart I, Vogel MW, Mariani J. Inhibition of Protein Kinase C (PKC) activity in cerebellar slice cultures rescues Lurcher Purkinje cells from pathological cell death. Program Number 169.12. Neuroscience Meeting Planner. Society for Neuroscience, 2007. Online. 2007.
Acknowledgments
This work was supported by an NSERC Discovery grant 400101 (C.L.A.) and NIH grant NS 34309 (M.W.V.)
Conflict of interest statement
There are no conflicts of interest for the authors of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armstrong, C.L., Duffin, C.A., McFarland, R. et al. Mechanisms of Compartmental Purkinje Cell Death and Survival in the Lurcher Mutant Mouse. Cerebellum 10, 504–514 (2011). https://doi.org/10.1007/s12311-010-0231-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-010-0231-4