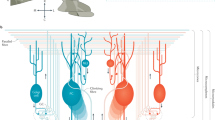
Abstract
The cerebellum is involved in learning and memory of sensory motor skills. However, the way this process takes place in local microcircuits is still unclear. The initial proposal, casted into the Motor Learning Theory, suggested that learning had to occur at the parallel fiber–Purkinje cell synapse under supervision of climbing fibers. However, the uniqueness of this mechanism has been questioned, and multiple forms of long-term plasticity have been revealed at various locations in the cerebellar circuit, including synapses and neurons in the granular layer, molecular layer and deep-cerebellar nuclei. At present, more than 15 forms of plasticity have been reported. There has been a long debate on which plasticity is more relevant to specific aspects of learning, but this question turned out to be hard to answer using physiological analysis alone. Recent experiments and models making use of closed-loop robotic simulations are revealing a radically new view: one single form of plasticity is insufficient, while altogether, the different forms of plasticity can explain the multiplicity of properties characterizing cerebellar learning. These include multi-rate acquisition and extinction, reversibility, self-scalability, and generalization. Moreover, when the circuit embeds multiple forms of plasticity, it can easily cope with multiple behaviors endowing therefore the cerebellum with the properties needed to operate as an effective generalized forward controller.




Similar content being viewed by others
Notes
Notes on the nature of models used to test the learning rules
In order to test the impact of the plasticity rules, they have been coupled to simplified cerebellar models [23] C. Casellato, A. Antonietti, J.A. Garrido, R.R. Carrillo, N.R. Luque, E. Ros, A. Pedrocchi, and E. D’Angelo [4]. Adaptive robotic control driven by a versatile spiking cerebellar network. PLoS One 9, e112265, [105] C. Casellato, A. Antonietti, J.A. Garrido, G. Ferrigno, E. D’Angelo, and A. Pedrocchi (2015). Distributed cerebellar plasticity implements generalized multiple-scale memory components in real-robot sensorimotor tasks. Front Comput Neurosci 9, 24. In the spiking cerebellar models, neurons are of the “integrate-and-fire” type, i.e., they have an RC membrane charging mechanism and a threshold for firing. The main properties of these neurons are to generate a linear frequency-intensity relationship in response to currents injected by synaptic inputs, to have a resting membrane potential or a basal firing frequency similar to real cells, and to show variations in firing during task processing reflecting the value ranges observed in vivo. Synaptic activation occurs through current injection into the model neurons and inputs from various neurons are integrated over the RC.
References
J Albus. The theory of cerebellar function. Math Biosci.1971;25-61.
Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–70.
Ito M, Kano M. "Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett. 1982;33:253–8.
D'Angelo E. The organization of plasticity in the cerebellar cortex: from synapses to control. Prog Brain Res. 2014;210:31–58.
Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci. 2012;13:619–35.
Hansel C, Linden DJ, D'Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci. 2001;4:467–75.
Kawato M, Gomi H. A computational model of four regions of the cerebellum based on feedback-error learning. Biol Cybern. 1992;68:95–103.
De Gruijl JR, Bazzigaluppi P, M.T. de Jeu. C.I. De Zeeuw. Climbing fiber burst size and olivary sub-threshold oscillations in a network setting. PLoS Comput Biol, (United States), 2012;e100. 2814.
Houk JC, Keifer J, Barto AG. Distributed motor commands in the limb premotor network. Trends Neurosci, (England). 1993;27-33.
Llinas R, Lang EJ, Welsh JP. The cerebellum, LTD, and memory: alternative views. Learn Mem. 1997;3:445–55.
Keating JG, Thach WT. Nonclock behavior of inferior olive neurons: interspike interval of Purkinje cell complex spike discharge in the awake behaving monkey is random. J Neurophysiol. 1995;73:1329–40.
Bengtsson F, Hesslow G. Cerebellar control of the inferior olive. Cerebellum (Norway). 2006; 7-14.
De Zeeuw CI, Simpson JI, Hoogenraad CC, Galjart N, Koekkoek SK, Ruigrok TJ. Microcircuitry and function of the inferior olive. Trends Neurosci (England). 1998;391-400.
Van Der Giessen RS, Koekkoek SK, van Dorp S, De Gruijl JR, Cupido A, Khosrovani S, et al. Role of olivary electrical coupling in cerebellar motor learning. Neuron. 2008;58:599–612.
D'Angelo E. Neural circuits of the cerebellum: hypothesis for function. Journal of Integr Neurosci. 2011. in press.
D'Angelo E, Mazzarello P, Prestori F, Mapelli J. , S. Solinas, P. Lombardo et al. The cerebellar network: From structure to function and dynamics. Brain Res Rev. 2010.
Ohtsuki G, Piochon C, Hansel C. Climbing fiber signaling and cerebellar gain control. Front Cell Neurosci. 2009;3:4.
Medina JF, Mauk MD. Simulations of cerebellar motor learning: computational analysis of plasticity at the mossy fiber to deep nucleus synapse. J Neurosci. 1999;19:7140–51.
Yang Y, Lisberger SG. Interaction of plasticity and circuit organization during the acquisition of cerebellum-dependent motor learning. Elife. 2013;2:e01574.
Hoffland BS, Bologna M, Kassavetis P, Teo JT, Rothwell JC, Yeo CH, et al. Cerebellar theta burst stimulation impairs eyeblink classical conditioning. J Physiol. 2012;590:887–97.
Monaco J, Casellato C, Koch G, D'Angelo E. Cerebellar theta burst stimulation dissociates memory components in eyeblink classical conditioning. Eur J Neurosci. 2014;40:3363–70.
Solinas S, Nieus T, D'Angelo E. A realistic large-scale model of the cerebellum granular layer predicts circuit spatio-temporal filtering properties. Front Cell Neurosci. 2010;4:12.
Casellato C, Antonietti A, Garrido JA, Carrillo RR, Luque NR, Ros E, et al. Adaptive robotic control driven by a versatile spiking cerebellar network. PLoS One. 2014;9:e112265.
Casellato C, Garrido JA, Franchin C, Ferrigno G, D’Angelo E, Pedrocchi A. Brain-inspired sensorimotor robotic platform: learning in cerebellum-driven movement tasks through a cerebellar realistic model. Challenges in Neuroengineering - SSCN - NCTA. 2013. Villamuora, Algarve - Portugal.
Casellato C, Pedrocchi A, Garrido JA, Luque NR, Ferrigno G, D'Angelo E et al. An integrated motor control loop of a human-like robotic arm: feedforward, feedback and cerebellum-based learning. Biomedical Robotics and Biomechatronics (BioRob), 2012 4th IEEE RAS & EMBS International Conference on. 2012;562-567.
Garrido JA, Luque NR, D'Angelo E, Ros E. Distributed cerebellar plasticity implements adaptable gain control in a manipulation task: a closed-loop robotic simulation. Front Neural Circuits. 2013;7:159.
Garrido JA, Ros E, D'Angelo E. Spike timing regulation on the millisecond scale by distributed synaptic plasticity at the cerebellum input stage: a simulation study. Front Comput Neurosci. 2013;7:64.
Luque NR, Garrido JA, Carrillo RR, D'Angelo E, Ros E. Fast convergence of learning requires plasticity between inferior olive and deep cerebellar nuclei in a manipulation task: a closed-loop robotic simulation. Front Comput Neurosci. 2014;8:97.
Mapelli L, Pagani M, Garrido JA, D'Angelo E (2015) .Integrated plasticity at inhibitory and excitatory synapses in the cerebellar circuit. Front. Cell. Neurosci. 9.
D'Angelo E, Rossi P, Armano S, Taglietti V. Evidence for NMDA and mGlu receptor-dependent long-term potentiation of mossy fiber-granule cell transmission in rat cerebellum. J Neurophysiol. 1999;81:277–87.
D'Angelo E, Rossi P, Gall D, Prestori F, Nieus T, Maffei A, et al. Long-term potentiation of synaptic transmission at the mossy fiber-granule cell relay of cerebellum. Prog Brain Res. 2005;148:69–80.
Sola E, Prestori F, Rossi P, Taglietti V, D'Angelo E. Increased neurotransmitter release during long-term potentiation at mossy fibre-granule cell synapses in rat cerebellum. J Physiol. 2004;557:843–61.
D'Errico A, Prestori F, D'Angelo E. Differential induction of bidirectional long-term changes in neurotransmitter release by frequency-coded patterns at the cerebellar input. J Physiol. 2009;587:5843–57.
Gall D, Prestori F, Sola E, D'Errico A, Roussel C, Forti L, et al. Intracellular calcium regulation by burst discharge determines bidirectional long-term synaptic plasticity at the cerebellum input stage. J Neurosci. 2005;25:4813–22.
Diwakar S, Lombardo P, Solinas S, Naldi G, D'Angelo E. Local field potential modeling predicts dense activation in cerebellar granule cells clusters under LTP and LTD control. PLoS ONE. 2011;6:e21928.
Roggeri L, Rivieccio B, Rossi P, D'Angelo E. Tactile stimulation evokes long-term synaptic plasticity in the granular layer of cerebellum. J Neurosci. 2008;28:6354–9.
Maffei A, Prestori F, Rossi P, Taglietti V, D'Angelo E. Presynaptic current changes at the mossy fiber-granule cell synapse of cerebellum during LTP. J Neurophysiol. 2002;88:627–38.
Maffei A, Prestori F, Shibuki K, Rossi P, Taglietti V, D'Angelo E. NO enhances presynaptic currents during cerebellar mossy fiber-granule cell LTP. J Neurophysiol. 2003;90:2478–83.
Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48.
Prestori F, Bonardi C, Mapelli L, Lombardo P, Goselink R, De Stefano ME, et al. Gating of long-term potentiation by nicotinic acetylcholine receptors at the cerebellum input stage. PLoS One. 2013;8:e64828.
Robberechts Q, Wijnants M, Giugliano M, De Schutter E. long-term depression at parallel fiber to golgi cell synapses. J Neurophysiol. 2010
Crepel F, Jaillard D. Protein kinases, nitric oxide and long-term depression of synapses in the cerebellum. Neuroreport. 1990;1:133–6.
Shibuki K, Okada D. Cerebellar long-term potentiation under suppressed postsynaptic Ca2+ activity. Neuroreport. 1992;3:231–4.
Qiu DL, Knöpfel T. An NMDA receptor/nitric oxide cascade in presynaptic parallel fiber-Purkinje neuron long-term potentiation. J Neurosci. 2007;27:3408–15.
Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron. 2004;44:691–700.
Lev-Ram V, Wong ST, Storm DR, Tsien RY. A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. Proc Natl Acad Sci U S A. 2002;99:8389–93.
Hartell NA. Parallel fiber plasticity. Cerebellum. 2002;1:3–18.
Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–47.
Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510.
Ito M. Neural design of the cerebellar motor control system. Brain Res. 1972;40:81–4.
Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102.
Ramakrishnan KB, D'Angelo E. Theta-Sensory input induced long-term potentiation (ltp) in purkinje cell layer of rat cerebellum. FENS Forum Neurosci (Barcelona, Spain) 2012.
Shen Y, Hansel C, Linden DJ. Glutamate release during LTD at cerebellar climbing fiber-Purkinje cell synapses. Nat Neurosci. 2002;5:725–6.
Bender VA, Pugh JR, Jahr CE. Presynaptically expressed long-term potentiation increases multivesicular release at parallel fiber synapses. J Neurosci. 2009;29:10974–8.
Soler-Llavina GJ, Sabatini BL. Synapse-specific plasticity and compartmentalized signaling in cerebellar stellate cells. Nat Neurosci. 2006;9:798–806.
Jörntell H, Ekerot CF. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron. 2002;34:797–806.
Kano M, Rexhausen U, Dreessen J, Konnerth A. Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature. 1992;356:601–4.
Bidoret C, Ayon A, Barbour B, Casado M. Presynaptic NR2A-containing NMDA receptors implement a high-pass filter synaptic plasticity rule. Proc Natl Acad Sci U S A. 2009;106:14126–31.
Casado M, Isope P, Ascher P. Involvement of presynaptic N-methyl-D-aspartate receptors in cerebellar long-term depression. Neuron. 2002;33:123–30.
Piochon C, Levenes C, Ohtsuki G, Hansel C. Purkinje cell NMDA receptors assume a key role in synaptic gain control in the mature cerebellum. J Neurosci. 2010;30:15330–5.
Rancillac A, Crépel F. Synapses between parallel fibres and stellate cells express long-term changes in synaptic efficacy in rat cerebellum. J Physiol. 2004;554:707–20.
Pugh JR, Raman IM. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron. 2006;51:113–23.
Pugh JR, Raman IM. Nothing can be coincidence: synaptic inhibition and plasticity in the cerebellar nuclei. Trends Neurosci. 2009;32:170–7.
Zhang W, Linden DJ. Long-term depression at the mossy fiber-deep cerebellar nucleus synapse. J Neurosci. 2006;26:6935–44.
Ouardouz M, Sastry B. Mechanisms underlying ltp of inhibitory synaptic transmission in the deep cerebellar nuclei. J Neurophysiol. 2000;84:1414–21.
Ouardouz M, Sastry BR. Mechanisms underlying LTP of inhibitory synaptic transmission in the deep cerebellar nuclei. J Neurophysiol. 2000;84:1414–21.
Morishita W, Sastry B. Postsynaptic mechanisms underlying long-term depression of gabaergic transmission in neurons of the deep cerebellar nuclei. J Neurophysiol. 1996;76:59–68.
D'Angelo E, De Zeeuw CI. Timing and plasticity in the cerebellum: focus on the granular layer. Trends Neurosci. 2009;32:30–40.
D'Angelo E, Mazzarello P, Prestori F, Mapelli J, Solinas S, Lombardo P, et al. The cerebellar network: from structure to function and dynamics. Brain Res Rev. 2011;66:5–15.
Bagnall MW, du Lac S. A new locus for synaptic plasticity in cerebellar circuits. Neuron. 2006;51:5–7.
Masuda N, Amari S. A computational study of synaptic mechanisms of partial memory transfer in cerebellar vestibulo-ocular-reflex learning. J Comput Neurosci. 2008;24:137–56.
Medina JF, Mauk MD. Computer simulation of cerebellar information processing. Nat Neurosci. 2000;3(Suppl):1205–11.
Armano S, Rossi P, Taglietti V, D'Angelo E. Long-term potentiation of intrinsic excitability at the mossy fiber-granule cell synapse of rat cerebellum. J Neurosci. 2000;20:5208–16.
Belmeguenai A, Hosy E, Bengtsson F, Pedroarena CM, Piochon C, Teuling E, et al. Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J Neurosci. 2010;30:13630–43.
Schreurs BG, Gusev PA, Tomsic D, Alkon DL, Shi T. Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. J Neurosci. 1998;18:5498–507.
Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci. 2000;3:109–11.
Zhang W, Shin JH, Linden DJ. Persistent changes in the intrinsic excitability of rat deep cerebellar nuclear neurones induced by EPSP or IPSP bursts. J Physiol. 2004;561:703–19.
Schweighofer N, Doya K, Lay F. Unsupervised learning of granule cell sparse codes enhances cerebellar adaptive control. Neuroscience. 2001;103:35–50.
Diwakar S, Magistretti J, Goldfarb M, Naldi G, D'Angelo E. Axonal Na + channels ensure fast spike activation and back-propagation in cerebellar granule cells. J Neurophysiol. 2009;101:519–32.
Arleo A, Nieus T, Bezzi M, D'Errico A, D'Angelo E, Coenen OJ. How synaptic release probability shapes neuronal transmission: information-theoretic analysis in a cerebellar granule cell. Neural Comput. 2010;22:2031–58.
Nieus T, Sola E, Mapelli J, Saftenku E, Rossi P, D'Angelo E. LTP regulates burst initiation and frequency at mossy fiber-granule cell synapses of rat cerebellum: experimental observations and theoretical predictions. J Neurophysiol. 2006;95:686–99.
Najafi F, Medina JF. Beyond "all-or-nothing" climbing fibers: graded representation of teaching signals in Purkinje cells. Front Neural Circuits. 2013;7:115.
Yang Y, Lisberger SG. Purkinje-cell plasticity and cerebellar motor learning are graded by complex-spike duration. Nature. 2014;510:529–32.
Lee KH, Mathews PJ, Reeves AM, Choe KY, Jami SA, Serrano RE, et al. Circuit mechanisms underlying motor memory formation in the cerebellum. Neuron. 2015;86:529–40.
Steuber V, Mittmann W, Hoebeek FE, Silver RA, De Zeeuw CI, Hausser M, et al. Cerebellar LTD and pattern recognition by Purkinje cells. Neuron. 2007;54:121–36.
D'Angelo E, Koekkoek SK, Lombardo P, Solinas S, Ros E, Garrido J, et al. Timing in the cerebellum: oscillations and resonance in the granular layer. Neuroscience. 2009;162:805–15.
Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108.
Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4:e179.
E. D'Angelo S. Casali. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Frontiers in Neural Circuits.2013;6.
Attwell PJE, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron. 2002;34:1011–20.
Cooke SF, Attwell PJ, Yeo CH. Temporal properties of cerebellar-dependent memory consolidation. J Neurosci. 2004;24:2934–41.
Attwell PJE, Rahman S, Yeo CH. Acquisition of eyeblink conditioning is critically dependent on normal function in cerebellar cortical lobule HVI. J Neurosci. 2001;21:5715–22.
De Zeeuw CI, Yeo CH. Time and tide in cerebellar memory formation. Curr Opin Neurobiol. 2005;15:667–74.
Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci. 2004;27:581–609.
Márquez-Ruiz J, Cheron G. Sensory stimulation-dependent plasticity in the cerebellar cortex of alert mice. PLoS One. 2012;7:e36184.
Yamazaki T, Tanaka S. Neural modeling of an internal clock. Neural Comput. 2005;17:1032–58.
Yamazaki T, Tanaka S. The cerebellum as a liquid state machine. Neural Netw. 2007;20:290–7.
Yamazaki T, Tanaka S. Computational models of timing mechanisms in the cerebellar granular layer. Cerebellum. 2009;8:423–32.
Masuda N, Amari S. A computational study of synaptic mechanisms of partial memory transfer in cerebellar vestibulo-ocular-reflex learning. J Comput Neurosci. 2008;24:137–56.
Pugh J, Raman I. Potentiation of mossy NMDA receptor activation followed by postinhibitory rebound current. Neuron. 2006;51:113–23.
Racine R, Wilson D, Gingell R, Sunderland D. Long-term potentiation in the interpositus and vestibular nuclei in the rat. Exp. Brain Res. 1986;63:158–62.
Yamazaki T, Nagao S. A computational mechanism for unified gain and timing control in the cerebellum. PLoS ONE. 2012;7:e33319.
Yamazaki T, Tanaka S. A spiking network model for passage-of-time representation in the cerebellum. Eur J Neurosci. 2007;26:2279–92.
Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–13.
Casellato C, Antonietti A, Garrido JA, Ferrigno G, D'Angelo E, Pedrocchi A. Distributed cerebellar plasticity implements generalized multiple-scale memory components in real-robot sensorimotor tasks. Front Comput Neurosci. 2015;9:24.
Conflict of Interest
The authors declare that there is no actual or potential conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’Angelo, E., Mapelli, L., Casellato, C. et al. Distributed Circuit Plasticity: New Clues for the Cerebellar Mechanisms of Learning. Cerebellum 15, 139–151 (2016). https://doi.org/10.1007/s12311-015-0711-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-015-0711-7