Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Li, Jian-Dong
Govardovskii, Victor I.
and
Steinberg, Roy H.
1994.
Light-dependent hydration of the space surrounding photoreceptors in the cat retina.
Visual Neuroscience,
Vol. 11,
Issue. 4,
p.
743.
MANGINI, NANCY J
HAUGH-SCHEIDT, LAURA
VALLE, JASON E
CRAGOE, JR, EDWARD J
RIPPS, HARRIS
and
KENNEDY, BRIAN G
1997.
Sodium-Calcium Exchanger in Cultured Human Retinal Pigment Epithelium.
Experimental Eye Research,
Vol. 65,
Issue. 6,
p.
821.
Kaneda, M.
Mochizuki, M.
Aoki, K.
and
Kaneko, A.
1997.
Modulation of GABAC Response by Ca2+ and Other Divalent Cations in Horizontal Cells of the Catfish Retina .
The Journal of General Physiology,
Vol. 110,
Issue. 6,
p.
741.
Gallemore, Ron P.
Hughes, Bret A.
and
Miller, Sheldon S.
1997.
Retinal pigment epithelial transport mechanisms and their contributions to the electroretinogram.
Progress in Retinal and Eye Research,
Vol. 16,
Issue. 4,
p.
509.
Baldridge, William H.
Kurennyi, Dmitri E.
and
Barnes, Steven
1998.
Calcium-Sensitive Calcium Influx in Photoreceptor Inner Segments.
Journal of Neurophysiology,
Vol. 79,
Issue. 6,
p.
3012.
Piccolino, Marco
Pignatelli, Angela
and
Rakotobe, Liramalala A.
1999.
Calcium-independent release of neurotransmitter in the retina: a “Copernican” viewpoint change.
Progress in Retinal and Eye Research,
Vol. 18,
Issue. 1,
p.
1.
Dmitriev, Andrey
Pignatelli, Angela
and
Piccolino, Marco
1999.
Resistance of Retinal Extracellular Space to Ca2+Level Decrease: Implications for the Synaptic Effects of Divalent Cations.
Journal of Neurophysiology,
Vol. 82,
Issue. 1,
p.
283.
Stalmans, P.
and
Himpens, B.
1999.
Properties of intra- and intercellular Ca2+-wave propagation elicited by mechanical stimulation in cultured RPE cells.
Cell Calcium,
Vol. 25,
Issue. 6,
p.
391.
Himpens, B.
Stalmans, P.
Gomez, P.
Malfait, M.
and
Vereecke, J.
1999.
Intra‐ and intercellular Ca
2+
signaling in retinal pigment epithelial cells during mechanical stimulation
.
The FASEB Journal,
Vol. 13,
Issue. 9001,
Crewther, David P.
2000.
The role of photoreceptors in the control of refractive state.
Progress in Retinal and Eye Research,
Vol. 19,
Issue. 4,
p.
421.
McBee, Joshua K
Palczewski, Krzysztof
Baehr, Wolfgang
and
Pepperberg, David R
2001.
Confronting Complexity: the Interlink of Phototransduction and Retinoid Metabolism in the Vertebrate Retina.
Progress in Retinal and Eye Research,
Vol. 20,
Issue. 4,
p.
469.
Malcolm, Andrew Todd
Kourennyi, Dmitri E.
and
Barnes, Steven
2003.
Protons and calcium alter gating of the hyperpolarization-activated cation current (Ih) in rod photoreceptors.
Biochimica et Biophysica Acta (BBA) - Biomembranes,
Vol. 1609,
Issue. 2,
p.
183.
Lavallee, C. R.
Chalifoux, J. R.
Moosally, A. J.
and
Balkema, G. W.
2003.
Elevated Free Calcium Levels in the Subretinal Space Elevate the Absolute Dark-Adapted Threshold in Hypopigmented Mice.
Journal of Neurophysiology,
Vol. 90,
Issue. 6,
p.
3654.
Cadetti, L.
Thoreson, W.B.
and
Piccolino, M.
2004.
Pre- and post-synaptic effects of manipulating surface charge with divalent cations at the photoreceptor synapse.
Neuroscience,
Vol. 129,
Issue. 3,
p.
791.
Linsenmeier, Robert A.
2005.
Neural Engineering.
p.
421.
ZHANG, DAO-QI
SUN, ZIYI
and
MCMAHON, DOUGLAS G.
2006.
Modulation of A-type potassium currents in retinal horizontal cells
by extracellular calcium and zinc.
Visual Neuroscience,
Vol. 23,
Issue. 5,
p.
825.
Arden, Geoffrey B.
and
Constable, Paul A.
2006.
The electro-oculogram.
Progress in Retinal and Eye Research,
Vol. 25,
Issue. 2,
p.
207.
Heiduschka, Peter
and
Schraermeyer, Ulrich
2008.
Comparison of visual function in pigmented and albino rats by electroretinography and visual evoked potentials.
Graefe's Archive for Clinical and Experimental Ophthalmology,
Vol. 246,
Issue. 11,
p.
1559.
Strauss, Olaf
2008.
Ocular Transporters In Ophthalmic Diseases And Drug Delivery.
p.
201.
Adijanto, Jeffrey
Banzon, Tina
Jalickee, Stephen
Wang, Nam S.
and
Miller, Sheldon S.
2009.
CO2-induced ion and fluid transport in human retinal pigment epithelium.
Journal of General Physiology,
Vol. 133,
Issue. 6,
p.
603.
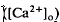 in the intact cat eye using ion-sensitive double-barreled microelectrodes. Two prominent changes in Ca2+ concentration were observed that differed in retinal location. There was a light-evoked increase in
in the intact cat eye using ion-sensitive double-barreled microelectrodes. Two prominent changes in Ca2+ concentration were observed that differed in retinal location. There was a light-evoked increase in 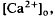 accompanied by brief ON and OFF transients, which was maximal in the inner plexiform layer and was not further studied. There was an unexpected sustained light-evoked decrease in
accompanied by brief ON and OFF transients, which was maximal in the inner plexiform layer and was not further studied. There was an unexpected sustained light-evoked decrease in 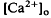 of relatively rapid onset and offset, which was maximal in the distalmost region of the subretinal space (SRS).
of relatively rapid onset and offset, which was maximal in the distalmost region of the subretinal space (SRS). 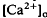 in the SRS was 1.0 mM higher than in the vitreous humor during dark adaptation and this transretinal gradient disappeared during rod-saturating illumination. After correcting for the light-evoked increase in the volume of the SRS, an increase in the total Ca2+ content of the SRS during illumination was revealed, which presumably represents the Ca2+ released by rods. To explain the light-evoked
in the SRS was 1.0 mM higher than in the vitreous humor during dark adaptation and this transretinal gradient disappeared during rod-saturating illumination. After correcting for the light-evoked increase in the volume of the SRS, an increase in the total Ca2+ content of the SRS during illumination was revealed, which presumably represents the Ca2+ released by rods. To explain the light-evoked 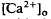 changes, we used the diffusion model described in the accompanying paper (Li et al., 1994b), with the addition of light-dependent sources of Ca2+ at the retina/retinal pigment epithelium (RPE) border and rod outer segments. We conclude that a drop in
changes, we used the diffusion model described in the accompanying paper (Li et al., 1994b), with the addition of light-dependent sources of Ca2+ at the retina/retinal pigment epithelium (RPE) border and rod outer segments. We conclude that a drop in 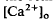 around photoreceptors, which persists during illumination and reduces a transretinal Ca2+ gradient, is the combined effect of the light-evoked SRS volume increase, Ca2+ release from photoreceptors, and an unidentified mechanism(s), which is presumably Ca2+ transport by the RPE. The relatively rapid onset and offset of the
around photoreceptors, which persists during illumination and reduces a transretinal Ca2+ gradient, is the combined effect of the light-evoked SRS volume increase, Ca2+ release from photoreceptors, and an unidentified mechanism(s), which is presumably Ca2+ transport by the RPE. The relatively rapid onset and offset of the 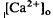 decrease remains unexplained. These steady-state shifts in
decrease remains unexplained. These steady-state shifts in 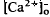 should have significant effects on photoreceptor function, especially adaptation.
should have significant effects on photoreceptor function, especially adaptation.



