Abstract
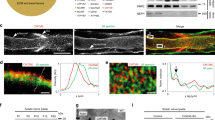
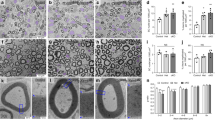
We examined the localization of Caspr and the K+ channels Kv1.1 and Kv1.2, all of which are intrinsic membrane proteins of myelinated axons in the PNS. Caspr is localized to the paranode; Kv1.1, Kv1.2 and their β2 subunit are localized to the juxtaparanode. Throughout the internodal region, a strand of Caspr staining is flanked by a double strand of Kv1.1/Kv1.2/Kvβ2 staining. This tripartite strand apposes the inner mesaxon of the myelin sheath, and forms a circumferential ring that apposes the innermost aspect of Schmidt-Lanterman incisures. The localization of Caspr and Kv1.2 are not disrupted in mice with null mutations of the myelin associated glycoprotein, connexin32, or Kv1.1 genes. At all of these locations, Caspr and Kv1.1/Kv1.2/Kvβ2 define distinct but interrelated domains of the axonal membrane that appear to be organized by the myelin sheath.
Similar content being viewed by others
References
Balice-gordon, R. J., Bone, L. J. & Scherer, S. S. (1998) Functional gap junctions in the Schwann cell myelin sheath. Journal of Cell Biology 142, 1095–1104.
Bellen, H. J., Lu, Y., Beckstead, R. & Bhat, M. A. (1998) Neurexin IV, Caspr and paranodinÑnovel members of the neurexin family: encounters of axons and glia. Trends in Neurosciences 21, 444–449.
Bennett, V., Lambert, S., Davis, J. Q. & Zhang, X. (1997) Molecular architecture of the specialized axonal membrane at the node of Ranvier. Society of General Physiology 52, 107–120.
Berthold, C.-H. & Rydmark, M. (1995) Morphology of normal peripheral axons. In The Axon (edited by Waxman, S. G., Kocsis, J. D. & Stys, P. K.), pp. 13–48. New York: Oxford University Press.
Bhat, M. A., Izaddoost, S., Lu, Y., Cho, K. O., Choi, K. W. & Bellen, H. J. (1999) Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell 96, 833–845.
Chiu, S. Y. (1991) Functions and distribution of voltagegated sodium and potassium channels in mammalian Schwann cells. Glia 4, 541–558.
Chiu, S. Y., Scherer, S. S., Blonski, M., Kang, S. S. & Messing, A. (1994) Axons regulate the expression of Shaker potassium channel genes in Schwann cells in peripheral nerve. Glia 12, 1–11.
Chiu, S. Y., Zhou, L., Zhang, C.-L. & Messing, A. (1999) Analysis of potassium channel functions in mammalian axons by gene knockouts. Journal of Neurocytology 28, 349–364.
Craven, S. E. & Bredt, D. S. (1998) PDZ proteins organize synaptic signaling pathways. Cell 93, 495–498.
Einheber, S., Zanazzi, G., Ching, W., Scherer, S. S., Milner, T. A., Peles, E. & Salzer, J. L. (1997) The axonal membrane protein Caspr/Neurexin IV is a component of the septate-like paranodal junctions that assemble during myelination. Journal of Cell Biology 139, 1495–1506.
Ellisman, M. H. & Levinson, S. R. (1982) Immunocytochemical localization of sodium channel distributions in the excitable membranes of. Electrophorus electricus. Proceedings of the National Academy of Sciences USA 79, 6707–6711.
Fannon, A. M., Sherman, D. L., Ilyinagragerova, G., Brophy, P. J., Friedrich, V. L. & Colman, D. R. (1995) Novel E-cadherin mediated adhesion in peripheral nerve: Schwann cell architecture is stabilized by autotypic adherens junctions. Journal of Cell Biology 129, 189–202.
Friede, R. L. & Samorajski, T. (1969) The clefts of Schmidt-Lantermann: A quantitative electron microscopic study of their structure in developing adult sciatic nerves of the rat. Anatomical Record 165, 89–102.
Gillespie, C. S., Sherman, D. L., Blair, G. E. & Brophy, P. J. (1994) Periaxin, a novel protein of myelinating Schwann cells with a possible role in axonal ensheathment. Neuron 12, 497–508.
Haimovich, B., Bonilla, E., Casadei, J. & Barchi, R. L. (1984) Immunocytochemical localization of the mammalian voltage-dependent sodium channel using polyclonal antibodies against the purified protein. Journal of Neuroscience 4, 2259–2268.
Hata, Y., Nakanishi, H. & Takai, Y. (1998) Synaptic PDZ domain-containing proteins. Neuroscience Research 32, 1–7.
Hirano, A. & Llena, J. F. (1995) Morphology of central nervous system axons. In The Axon (edited by Waxman, S. G., Kocsis, J. D. & Stys, P. K.), pp. 49–67. New York: Oxford University Press.
Hopkins, W. F., Allen, M. L., Mouamed, K. M. & Tempel, B. L. (1994) Properties of voltage-gated K+ currents expressed in Xenopus oocytes by mKv1.1, mKv1.2 and their heteromultimers as revealed by mutagenesis of the dendrotoxin-binding site in Kv1.1. Pflügers Archives 428, 382–390.
Ichimura, T. & Ellisman, M. H. (1991) Threedimensional fine structure of cytoskeletal-membrane interactions at nodes of Ranvier. Journal of Neurocytology 20, 667–681.
Kim, E., Niethammer, M., Rothschild, A., Jan, Y. N. & Sheng, M. (1995) Clustering of Shakertype K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature 378, 85–88.
Lambert, S., Davis, J. Q. & Bennett, V. (1997) Morphogenesis of the node of Ranvier: co-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates. Journal of Neuroscience 17, 7025–7036.
Lee, V., Wu, H. L. & Schlaepfer, W. W. (1982) Monoclonal antibodies recognized individual neurofilament triplet proteins. Proceedings of the National Academy of Sciences USA 79, 6089–6092.
Li, C., Topak, M. B., Gerial, R., Calpoff, S., Abramow-newerly, W., Trapp, B., Peterson, A. & Roder, J. (1994) Myelination in the absence of myelin-associated glycoprotein. Nature 369, 747–750.
Li, C., Trapp, B., Ludwin, S., Peterson, A. & Roder, J. (1998) Myelin associated glycoprotein modulates glia-axon contact in vivo. Journal of Neuroscience Research 51, 210–217.
Martini, R. & Schachner, M. (1988) Immunoelectron microscopic localization of neural cell adhesion molecules (L1,N-CAM,and myelin-associated glycoprotein) in regenerating adult mouse sciatic nerve. Journal of Cell Biology 106, 1735–1746.
Mata, M., Kupina, N. & Fink, D. J. (1992) Phosphorylation-dependent neurofilament epitopes are reduced at the node of Ranvier. Journal of Neurocytology 21, 199–210.
Menegoz, M., Gaspar, P., Le Bert, M., Galvez, T., Burgaya, F., Palfrey, C., Ezan, P., Arnos, F. & Girault, J.-A. (1997) Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron 19, 319–331.
Mi, H. Y., Deerinck, T. J., Ellisman, M. H. & Schwarz, T. L. (1995) Differential distribution of closely related potassium channels in rat Schwann cells. Journal of Neuroscience 15, 3761–3774.
Miller, R. G. & Da Silva, P. P. (1977) Particle rosettes in the periaxonal Schwann cell membrane and particle clusters in the axolemma of rat sciatic nerve. Brain Research 130, 135–141.
Montag, D., Giese, K. P., Bartsch, U., Martini, R., Lang, Y., Bluthmann, H., Karthigasan, J., Kirschner, D. A., Wintergerst, E. S., Nave, K.-A., Zielasek, J., Toyka, K. V., Lipp, H.-P. & Schachner, M. (1994) Mice deficient for the myelinassociated glycoprotein show subtle abnormalities in myelin. Neuron 13, 229–246.
Nelles, E., Butzler, C., Jung, D., Temme, A., Gabriel, H.-D., Dahl, U., Traub, O., Stumpel, F., Jungermann, K., Zielasek, J., Toyka, K. V., Dermietzel, R. & Willecke, K. (1996) Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proceedings of the National Academy of Sciences USA 93, 9565–9570.
Pedraza, L., Owens, G. C., Green, L. A. D. & Salzer, J. L. (1990) The myelin-associated glycoproteins: membrane disposition, evidence of a novel disulfide linkage between immunoglobulin-like domains, and posttranslational palmitylation. Journal of Cell Biology 111, 2651–2661.
Peles, E., Nativ, M., Lustig, M., Grumet, M., Martinez, R., Plowman, G. D. & Schlessinger, J. (1997) Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. European Molecular Biology Organization Journal 16, 978–988.
Peters, A., Palay, S. L. & Webster, H. D. (1991) . The Fine Structure of the Nervous System. New York: Oxford University Press.
Raine, C. S. (1982) Differences between the nodes of Ranvier of large and small diameter fibres in the P.N.S. Journal of Neurocytology 11, 935–947.
Rasband, M., Trimmer, J. S., Schwarz, T. L., Levinson, S. R., Ellisman, M. H., Schachner, M. & Shrager, P. (1998) Potassium channel distribution, clustering, and function in remyelinating rat axons. Journal of Neuroscience 18, 36–47.
Rhodes, K. J., Strassle, B. W., Monaghan, M. M., Bekele-arcuri, Z., Matos, M. F. & Trimmer, J. S. (1997) Association and colocalization of the Kvβ1 and Kvβ2 in with Kv1β-subunits in mammalian brain K+ channel complexes. Journal of Neuroscience 17, 8646–8258.
Rosenbluth, J. (1976) Intramembranous particle distribution at the node of Ranvier and adjacent axolemma in myelinated axons of the frog brain. Journal of Neurocytology 5, 731–745.
Rosenbluth, J. (1988) Role of glial cells in the differentiation and function of myelinated axons. International Journal of Developmental Neuroscience 6, 3–24.
Salzer, J. L. (1997) Clustering sodium channels at the node of Ranvier: Close encounters of the axon-glia kind. Neuron 18, 843–846.
Scherer, S. S. (1996) Molecular specializations at nodes and paranodes in peripheral nerve. Microscopy Research and Technique 34, 452–461.
Scherer, S. S., Xu, Y.-T., Nelles, E., Fischbeck, K., Willecke, K. & Bone, L. J. (1998) Connexin32-null mice develop a demyelinating peripheral neuropathy. Glia 24, 8–20.
Sheng, M., Tsaur, M.-L., Jan, Y. N. & Jan, L. Y. (1994) Contrasting subcellular localization of the Kv1.2 K+ channel subunit in different neurons of rat brain. Journal of Neuroscience 14, 2408–2417.
Shrager, P. & Wu, J. V. (1994) Ionic channels of myelinated axons. In Handbook of Membrane Channels, pp. 105–113. Academic Press.
Small, J. R., Ghabriel, M. N. & Allt, G. (1987) The development of Schmidt-Lanterman incisures: an electron microscope study. Journal of Anatomy 150, 277–286.
Smart, S. L., Lopantsev, V., Zhang, C. L., Robbins, C. A., Wang, H., Chiu, S. Y., Schwartzkroin, P. A., Messing, A. & Tempel, B. L. (1998) Deletion of the Kv1.1 potassium channel causes epilepsy in mice. Neuron 20, 809–819.
Srinivasan, Y., Schachner, M. & Catterall, W. (1998) Interaction of voltage-gated sodium channels with the extracellular matrix moleculres tenascin-C and tenascin-R. Proceedings of the National Academy of Sciences USA 95, 15753–15757.
Stolinski, C., Breathnach, A. S., Martin, B., Thomas, P. K., King, R. M. H. & Gabriel, G. (1981) Associated particle aggregates in juxtaparanodal axolemma and adaxonal Schwann cell membrane of rat peripheral nerve. Journal of Neurocytology 10, 679–691.
Stolinski, C., Breathnach, A. S., Thomas, P. K., Gabriel, G. & King, R. M. H. (1985) Distribution of particle aggregates in the internodal axolemma and adaxonal Schwann cell membrane of rodent peripheral nerve. Journal of the Neurological Sciences 67, 213–222.
Tao-cheng, J.-H. & Rosenbluth, J. (1984) Extranodal particle accumulations in the axolemma of myelinated frog optic axons. Brain Research 308, 289–300.
Tejedor, F. J., Bokhari, A., Rogero, O., Gorczyca, M., Zhang, J., Kim, E., Sheng, M. & Budnik, V. (1997) Essential role for dlg in synaptic clustering of Shaker K+ channels. in vivo. Journal of Neuroscience 17, 152–159.
Topinka, J. R. & Bredt, D. S. (1998) N-terminal palmitoylation of PSD-95 regulates association with cell membranes and interaction with K+ channel Kv1.4. Neuron 20, 125–134.
Trapp, B. D. & Quarles, R. H. (1982) Presence of the myelin-associated glycoprotein correlates with alterations in the periodicity of peripheral myelin. Journal of Cell Biology 92, 877–882.
Vabnick, I., Messing, A., Chiu, S. Y., Levinson, S. R., Schachner, M., Roder, J., Li, C. M., Novakovic, S. & Shrager, P. (1997) Sodium channel distribution in axons of hypomyelinated and MAG null mutant mice. Journal of Neuroscience Research 50, 321–336.
Vabnick, I. & Shrager, P. (1998) Ion channel redistribution and function during development of the myelinated axon. Journal of Neurobiology 37, 80–96.
Vabnick, I., Trimmer, J. S., Schwarz, T. L., Levinson, S. R., Risal, D. & Shrager, P. (1999) Dynamic potassium channel distributions during axonal development prevent aberrant firing patterns. Journal of Neuroscience 19, 747–758.
Veh, R. W., Lichtinghagen, R., Sewing, S., Wunder, F., Grumbach, I. M. & Pongs, O. (1995) Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. European Journal of Neuroscience 7, 2189–2205.
Wang, H., Kunkel, D. D., Martin, T. M., Schwartkroin, P. A. & Tempel, B. L. (1993) Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature 365, 75–79.
Wang, H., Kunkel, D. D., Schwartzkroin, P. A. & Tempel, B. L. (1994) Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. Journal of Neuroscience 14, 4588–4599.
Yin, X. H., Crawford, T. O., Griffin, J. W., Tu, P. H., Lee, V. M. Y., Li, C. M., Roder, J. & Trapp, B. D. (1998) Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. Journal of Neuroscience 18, 1953–1962.
Zamboni, L. & De martino, C. (1967) Buffered picricacid formaldehyde: a new rapid fixative for electronmicroscopy. Journal of Cell Biology 35, 148A.
Zhou, L., Zhang, C. L., Messing, A. & Chiu, S. Y. (1998) Temperature-sensitive neuromuscular transmission in Kv1.1 null mice: Role of potassium channels under the myelin sheath in young nerves. Journal of Neuroscience 18, 7200–7215.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arroyo, E.J., Xu, YT., Zhou, L. et al. Myelinating Schwann cells determine the internodal localization of Kv1.1, Kv1.2, Kvβ2, and Caspr. J Neurocytol 28, 333–347 (1999). https://doi.org/10.1023/A:1007009613484
Issue Date:
DOI: https://doi.org/10.1023/A:1007009613484