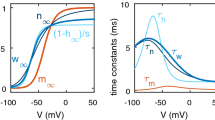
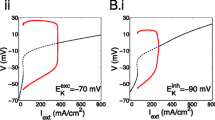
Abstract
Modulation of extracellular potassium concentration ([K]o) has a profound impact on the excitability of neurons and neuronal networks. In the CA3 region of the rat hippocampus synchronized epileptiform bursts occur in conditions of increased [K]o. The dynamic nature of spontaneous neuronal firing in high [K]o is therefore of interest. One particular interest is the potential presence of bistable behaviors such as the coexistence of stable repetitive firing and fixed rest potential states generated in individual cells by the elevation of [K]o. The dynamics of repetitive activity generated by increased [K]o is investigated in a 19-compartment hippocampal pyramidal cell (HPC) model and a related two-compartment reduced HPC model. Results are compared with those for the Hodgkin-Huxley equations in similar conditions. For neural models, [K]o changes are simulated as a shift in the potassium reversal potential (E K ). Using phase resetting and bifurcation analysis techniques, all three models are shown to have specific regions of E K that result in bistability. For activity in bistable parameter regions, stimulus parameters are identified that switch high-potassium model behavior from repetitive firing to a quiescent state. Bistability in the HPC models is limited to a very small parameter region. Consequently, our results suggest that it is likely some HPCs in networks exposed to high [K]o continue to burst such that a stable, quiescent network state does not exist. In [K]o ranges where HPCs are not bistable, the population may still exhibit bistable behaviors where synchronous population events are reversibly annihilated by phase resetting pulses, suggesting the existence of a nonsynchronous network attractor.
Similar content being viewed by others
References
Aihara K, Matsumoto G (1983) Two stable steady states in the Hodgkin-Huxley axons. Biophysiol. J. 41:87-89.
Best EN (1979) Null space in the Hodgkin-Huxley equations: A critical test. Biophysiol. J. 27:87-104.
Bikson M, Ghai RS, Baraban SC, Durand DM (1999) Modulation of burst frequency, duration, and amplitude in the zero-Ca(2+) model of epileptiform activity. J. Neurophysiol. 82:2262-2270.
Booth V, Rinzel J (1995) A minimal, compartmental model for a dendritic origin of bistability of motoneuron firing patterns. J. Comput. Neurosci. 2:299-312.
Chay TR, Lee S (1984) Impulse responses of automaticity in the Purkinje fiber. Biophysiol. J. 45:841-849.
Clay JR (1998) Excitability of the squid giant axon revisited. 80:903-913.
Dietzel I, Heinemann U, Lux HD (1989) Relations between slow extracellular potential changes, glial potassium buffering, and electrolyte and cellular volume changes during neuronal hyperactivity in cat brain. Glia 2:25-44.
Doedel EJ, Kernevez JP (1986) Software for Continuation Problems in Ordinary Differential Equations with Applications. California Institute of Technology, Pasadena.
Durand DM, Warman WN (1994) Desynchronization of neuronal activity by extracellular current pulses in the hippocampus in vitro. J. Physiol. (London) 480:527-537.
Ermentrout B (1997) <http://www.pitt.edu/~phase/>
Glass L, Winfree AT (1984) Discontinuities in phase-resetting experiments. Am. J. Physiol. 246:R251-R258.
Guckenheimer J, Labouriau IS (1993) Bifurcation of the Hodgkin and Huxley equations: A new twist. Bull. Math Biol. 55:937-952.
Guttman R, Lewis S, Rinzel J (1980) Control of repetitive firing in squid axon membrane as a model for neuroneoscillator. J. Physiol. (London) 305:377-395.
Heineman U, Lux HD, Gutnick MJ (1977) Extracellular free calcium and potassium during paroxysmal activity in the cerebral cortex of the cat. Exp. Brain Res. 27:237-243.
Hille B (1992) Ionic Channels of Excitable Membranes. Sinauer Associates.
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation of nerve. J. Physiol. (London) 117:500-544.
Holden AV, Yoda M (1981) Ionic channel density of excitable membranes can act as a bifurcation parameter. Biol. Cybern. 42:29-38.
Izhikevich EM (2000) Neural excitability, spiking and bursting. Int. J. Bif. Chaos 10:1171-1266.
Jahangiri A (1998). Phase resetting of high potassium epileptiform activity in the Ca3 region of rat hippocampal slices. Ph.D. thesis, Case Western Reserve University, Cleveland, OH.
Jahangiri A, Durand DM, Lin JC (1997) Singular stimulus parameters to annihilate spontaneous activity in the Hodgkin-Huxley model with elevated potassium (Abstract). IEEE/EMBS Proceedings.
Jalife J, Antzelevitch C (1979) Phase resetting and annihilation of pacemaker activity in cardiac tissue. Science 206:695-697.
Jefferys JG (1995) Nonsynaptic modulation of neuronal activity in the brain: Electric currents and extracellular ions. Physiol. Rev. 75:689-723.
Jensen MS, Azouz R, Yaari Y (1994) Variant firing patterns in rat hippocampal pyramidal cells modulated by extracellular potassium. J. Neurophysiol. 71:831-839.
Jensen MS, Yaari Y (1988) The relationship between interictal and ictal paroxysms in an in vitro model of focal hippocampal epilepsy. Ann. Neurol. 24:591-598.
Jensen MS, Yaari Y (1997) Role of intrinsic burst firing, potassium accumulation, and electrical coupling in the elevated potassium model of hippocampal epilepsy. J. Neurophysiol. 77:1224-1233.
Korn SJ, Giacchino JL, Chamberlin NL, Dingledine R (1987) Epileptiform burst activity induced by potassium in the hippocampus and its regulation by gaba-mediated inhibition. J. Neurophysiol. 57:325-341.
McBain CJ, Traynelis SF, Dingledine R (1993) High potassium-induced synchronous bursts and electrographic seizures. In: Schwartzkroin P., ed. Epilepsy: Models, Mechanisms and Concepts. Cambridge University Press, Cambridge, pp. 437-461.
Mines GR (1914) On circulating excitation on heart muscles and their possible relation to tachycardia and fibrillation. Trans. R. Soc. Can. 4:43-53.
Pinsky P (1994) Mathematical models of hippocampal neurons and neural networks: Exploiting multiple time scales. Ph.D. Thesis, University of Maryland.
Pinsky P, Rinzel J (1994) Intrinsic and network rhythmogenesis in a reduced Traub model for Ca3 neurons. J. Comput. Neurosci. 1:39-69.
Rinzel J (1985) Excitation dynamics: Insights from simplified membrane models. Federation Proc. 44:2944-2946.
Rinzel J, Ermentrout GB (1989) Analysis of neural excitability and oscillations. In: Koch C, Seger I, eds. Methods in Neuronal Modeling: From Synapses To Networks. MIT Press, Cambridge, MA. pp. 135-169.
Rutecki PA, Lebeda FJ, Johnston D (1985) Epileptiform activity induced by changes in extracellular potassium in hippocampus. J. Neurophysiol. 54:1363-1374.
Traub RD, Dingledine R (1990) Model of synchronized epileptiform bursts induced by high potassium in Ca3 region of rat hippocampal slice: Role of spontaneous epsps in initiation. J. Neurophysiol. 64:1009-1018.
Traub RD, Miles R (1991a) Multiple modes of neuronal population activity emerge after modifying specific synapses in a model of the Ca3 region of the hippocampus. Ann. N.Y. Acad. 627:277-290.
Traub RD, Miles R (1991b) Neuronal Networks of the Hippocampus. Cambridge University Press, Cambridge.
Traub RD, Wong RK, Miles R, Michelson H (1991) A model of a Ca3 hippocampal pyramidal neuron incorporating voltageclamp data on intrinsic conductances. J. Neurophysiol. 66:635-650.
Traynelis SF, Dingledine R (1988) Potassium-induced spontaneous electrographic seizures in the rat hippocampal slices. J. Neurophysiol. 59:259-276.
Williams SR, Toth TI, Turner JP, Hughes SW, Crunelli V (1997) The “window” component of the low threshold Ca2+ current produces input signal amplification and bistability in cat and rat thalamocortical neurons. J. Physiol. 505:689-705.
Wilson HR, Cowan JD (1972) Excitatory and inhibitory interactions in localized populations of model neurons. Biophys. J. 12:1-24.
Winfree AT (1987) When Time Breaks Down. Princeton University Press, Princeton, NJ.
Winfree AT (1980) The Geometry of Biological Time. Springer-Verlag, New York.
Wong, RKS, Traub RD, Miles R (1986) Cellular basis of neuronal synchrony in epilepsy. Adv. Neurol. 44:583-592.
Zipes DP (1975) Electrophysiological mechanisms involved in ventricular fibrillation. Circulation 52:III120-130.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hahn, P.J., Durand, D.M. Bistability Dynamics in Simulations of Neural Activity in High-Extracellular-Potassium Conditions. J Comput Neurosci 11, 5–18 (2001). https://doi.org/10.1023/A:1011250329341
Issue Date:
DOI: https://doi.org/10.1023/A:1011250329341