Abstract
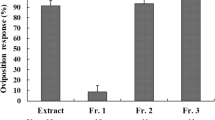
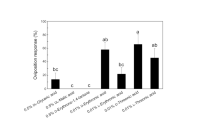
Female butterflies of the spicebush swallowtail, Papilio troilus, are specialists, ovipositing on plants in the family Lauraceae. Column chromatography and HPLC were used to isolate an oviposition stimulant from the leaves of one of its hosts, Sassafras albidum. The stimulant was identified as 3-trans-caffeoyl-muco-quinic acid on the basis of FAB-MS and 1H NMR spectra as compared to a compound previously isolated from another plant. It was not active alone, but it increased the oviposition activity of butterflies when combined with other stimulant(s) at a concentration of 7 ng/mm2 leaf surface area. Other caffeoylquinic acid isomers tested did not have this effect. This is the first report of a swallowtail contact oviposition stimulant from a plant in the family Lauraceae.
Similar content being viewed by others
REFERENCES
Brandl, W., and Herrmann, K. 1983. Analytical and preparative high-performance liquid chromatography of hydroxycinnamic acid esters. J. Chromatogr. 260:447-455.
Carter, M. E., and Feeny, P. P. 1985. Techniques for maintaining a culture of the black swallowtail butterfly, Papilio polyxenes asterius Stoll (Papilionidae). J. Lepid. Soc. 39:125-133.
Carter, M., Sachdev-Gupta, K., and Feeny, P. 1998. Tyramine isolated from parsnip leaves: A stimulant and synergist for oviposition of the black swallowtail. Physiol. Entomol. 23:303-312.
Cronquist, A. 1981. An Integrated System of Classification of Flowering Plants, Columbia University Press, New York.
Dethier, V. G. 1941. Chemical factors determining the choice of food plants by Papilio larvae. Am. Nat. 75:61-73.
Feeny, P. 1991. Chemical constraints on the evolution of swallowtail butterflies, pp. 315-340, in P. W. Price, T. M. Lewinsohn, G. W. Fernandes, and W. W. Benson (eds.). Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. John Wiley & Sons, New York.
Feeny, P. 1995. Ecological opportunism and chemical constraints on the host associations of swallowtail butterflies, pp. 9-15, in J. M. Scriber, Y. Tsubaki, and R. C. Lederhouse (eds.). Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Scientific Publishers, Gainesville, Florida.
Feeny, P., Rosenberry, L., and Carter, M. 1983. Chemical aspects of oviposition behavior in butterflies, pp. 27-76, in S. Ahmad (ed.). Herbivorous Insects: Host-Seeking Behavior and Mechanisms. Academic Press, New York.
Feeny, P., Sachdev, K., Rosenberry, L., and Carter, M. 1988. Luteolin 7-O-(6″-O-malonyl)-β-D-glucoside and trans-chlorogenic acid: Oviposition stimulants for the black swallowtail butterfly. Phytochemistry 27:3439-3448.
Hagen, R. H., and Scriber, J. M. 1991. Systematics of the Papilio glaucus and P. troilus species groups (Lepidoptera: Papilionidae): Inferences from allozymes. Ann. Entomol. Soc. Am. 84:380-395.
Haribal, M., Feeny, P., and Lester, C. 1998. A caffeoylcyclohexane-1-carboxylic acid derivative from Asimina triloba. Phytochemistry 49:103-108.
Honda, K. 1986. Flavanone glycosides as oviposition stimulants in a papilionid butterfly, Papilio protenor. J. Chem. Ecol. 12:1999-2010.
Honda, K. 1990. Identification of host-plant chemicals stimulating oviposition by swallowtail butterfly, Papilio protenor. J. Chem. Ecol. 16:325-337.
Howe, W. H. 1975. The Butterflies of North America. Doubleday, Garden City, New York.
Klots, A. B. 1951. A Field Guide to the Butterflies of North America East of the Great Plains. Houghton Mifflin, Boston.
Lederhouse, R. C., Ayres, M. P., and Scriber, J. M. 1990. Adult nutrition affects male virility in Papilio glaucus L. Funct. Ecol. 4:743-751.
Miller, J. S. 1987. Host-plant relationships in the Papilionidae (Lepidoptera): Parallel cladogenesis or colonization? Cladistics 3:105-120.
MÖller, B., and Herrmann, K. 1982. Analysis of quinic acid esters of hydroxycinnamic acids in plant material by capillary gas chromatography and high-performance liquid chromatography. J. Chromatogr. 241:371-379.
MÖller, B., and Herrmann, K. 1983. Quinic acid esters of hydroxycinnamic acids in stone and pome fruit. Phytochemistry 22:477-481.
Morishita, H., Iwahashi, H., Osaka, N., and Kido, R. 1984. Chromatographic separation and identification of naturally occurring chlorogenic acids by 1H nuclear magnetic resonance spectroscopy and mass spectrometry. J. Chromatogr. 315:253-260.
Munroe, E. M. 1961. The classification of the Papilionidae (Lepidoptera). Can. Entomol. Suppl. 17:1-51.
Nagels, L., Van Dongen, W., De Brucker, J., and De Pooter, H. 1980. High-performance liquid chromatographic separation of naturally occurring esters of phenolic acids. J. Chromatogr. 187:181-187.
Nishida, R. 1989. Flavonoid-mediated host recognition by swallowtail butterflies, pp. 199-212, in N. P. Das (ed.). Flavonoids in Biology and Medicine. National University of Singapore, Singapore.
Nishida, R. 1995. Oviposition stimulants of swallowtail butterflies, pp. 17-26, in J. M. Scriber, Y. Tsubaki, and R. C. Lederhouse (eds.). Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Scientific Publishers, Gainesville, Florida.
Nishida, R., and Fukami, H. 1989. Oviposition stimulants of an Aristolochia-feeding swallowtail butterfly, Atrophaneura alcinous. J. Chem. Ecol. 15:2565-2575.
Nishida, R., Ohsugi, S., Kokubo, S., and Fukami, H. 1987. Oviposition stimulants of a Citrus-feeding swallowtail butterfly, Papilio xuthus L. Experientia 43:342-344.
Nishida, R., Ohsugi, T., and Fukami, H. 1990. Oviposition stimulant activity of tryptamine analogs of a Rutaceae-feeding swallowtail butterfly, Papilio xuthus. Agric. Biol. Chem. 54:1853-1855.
Nitao, J. K., Ayres, M. P., Lederhouse, R. C., and Scriber, J. M. 1991. Larval adaptation to lauraceous hosts: Geographic divergence in the spicebush swallowtail butterfly. Ecology 72:1428-1435.
Ohsugi, T. 1991. Activity of oviposition stimulant analogs for a Rutaceae-feeding swallowtail butterfly, Papilio xuthus. Bull. Wakayama Med. Coll. 21:5-9.
Ohsugi, T., Nishida R., and Fukami, H. 1991. Multicomponent system of oviposition stimulants for a Rutaceae-feeding swallowtail butterfly, Papilio xuthus. App. Entomol. Zool. 26:29-40.
Scott, J. A. 1986. The Butterflies of North America, Stanford University Press, Stanford.
Scriber, J. M. 1984. Larval foodplant utilization by the world Papilionidae (Lep.): Latitudinal gradients reappraised. Tokurana (Acta Rhopalocerlogica) 6/7:1-50.
Scriber, J. M., Lederhouse, R. C., and Hagen, R. H. 1991. Foodplants and evolution within Papilio glaucus and Papilio troilus species groups (Lepidoptera: Papilionidae), pp. 341-373, in P. W. Price, T. M. Lewinsohn, G. W. Fernandes, and W. W. Benson (eds.). Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. John Wiley & Sons, New York.
Sperling, F. A. 1993. Mitochondrial DNA variation and Haldane's rule in the Papilio glaucus and P. troilus species groups. Heredity 71:227-233.
Stevenson, P. C., Anderson, J. C., Blaney, W. M., and Simmonds, M. S. J. 1993. Developmental inhibition of Spodoptera litura (Fab.) larvae by a novel caffeoylquinic acid from the wild groundnut, Arachis paraguariensis (Chod et Hassl.). J. Chem. Ecol. 19:2917-2933.
Thorne, R. F. 1992. Classification and geography of the flowering plants. Bot. Rev. 58:225-348.
Tyler, H., and Brown, K. S. J. 1994. Swallowtail Butterflies of the Americas. Scientific Publishers, Gainesville, Florida.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carter, M., Feeny, P. & Haribal, M. An Oviposition Stimulant for Spicebush Swallowtail Butterfly, Papilio troilus, from Leaves of Sassafras albidum . J Chem Ecol 25, 1233–1245 (1999). https://doi.org/10.1023/A:1020962422712
Issue Date:
DOI: https://doi.org/10.1023/A:1020962422712