Abstract
Purpose. Racemic valnoctamide (VCD) is a central nervous system- active drug commercially available in Europe. VCD possesses two chiral centers and, therefore, it exists as a mixture of four stereoisomers. The purpose of this study was to evaluate the anticonvulsant activity of two VCD stereoisomers in comparison with VCD (racemate), valpromide (VPD), and valproic acid (VPA) and to study their pharmacokinetic-pharmacodynamic relationships.
Methods. The ability of racemic VCD, (2S,3S)-VCD, (2R,3S)-VCD and VPD to block partial seizures was studied in the 6Hz psychomotor seizure model in mice and in the hippocampal kindled rat. The ability of (2S,3S)-VCD and (2R,3S)-VCD to prevent generalized seizures was evaluated in the maximum electroshock (MES) and subcutaneous metrazole (sc Met) seizure tests. The PK of (2S,3S)-VCD, (2R,3S)-VCD, and VPD was studied in the mice utilized in the 6Hz model.
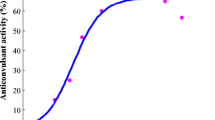
Results. All of the tested compounds were effective in the models tested. No significant difference in ED50 values was observed but the plasma and brain EC50 values of (2R,3S)-VCD in the 6Hz model at 32 mA stimulation were 2-fold higher than the EC50 values of (2S,3S)-VCD. An excellent pharmacokinetic-pharmacodynamic correlation was found between the plasma and brain concentrations of the VCD stereoisomers and their anticonvulsant effect in mice. Stereoselectivity was observed in clearance, volume of distribution, and in brain-to-plasma AUC ratio at a dose of 25 mg/kg, but the difference disappeared at higher doses as the clearance of the stereoisomers decreased and their half-life increased. For (2R,3S)-VCD the brain-to-plasma AUC ratio doubled at the tested dose range, while it remained constant for (2S,3S)-VCD.
Conclusions. Racemic VCD, VPD, (2R,3S)-VCD, and (2S,3S)-VCD are effective anticonvulsants in animal models of partial seizures and are more potent than VPA. The more favorable brain penetration of (2S,3S)-VCD and its lower EC50 value in the 6Hz test provides one advantage over (2R,3S)-VCD as a new antiepileptic drug.
Similar content being viewed by others
REFERENCES
W. Stepansky. A clinical study in the use of valmethamide, an anxiety reducing drug. Curr. Ther. Res. 2:144-147 (1960).
J. P. Chambon and A. Perio. Valnoctamide: pharmacological data suggesting anticonvulsant activity. Neurosci. Lett. S5:327(1980).
A. Haj-Yehia and M. Bialer. Structure-pharmacokinetic relationships in a series of valpromide derivatives with antiepileptic activity. Pharm. Res. 6:683-689 (1989).
H. Lindekens, I. Smolders, G. M. Khan, M. Bialer, G. Ebinger, and Y. Michotte. In vivo study of the effects of valpromide and valnoctamide in the pilocarpine rat model of focal epilepsy. Pharm. Res. 17:1408-1413 (2000).
M. Roeder, O. Spiegelstein, V. Schurig, M. Bialer, and B. Yagen. Absolute configuration of the four stereoisomers of valnoctamide (2-ethyl-3-methyl valeramide), a potentially new stereospecific antiepileptic and CNS drug. Tetrahedron Asymm. 10:841-853 (1999).
S. Barel, B. Yagen, V. Schurig, S. Soback, F. Pisani, E. Perucca, and M. Bialer. Stereoselective pharmacokinetic analysis of valnoctamide in healthy subjects and in patients with epilepsy. Clin. Pharmacol. Ther. 61:442-449 (1997).
O. Spiegelstein, B. Yagen, G. Bennett, R. H. Finnell, S. Blotnik, and M. Bialer. Stereoselective pharmacokinetic analysis of valnoctamide-a CNS-active chiral amide analogue of valproic acid in dogs, rats and mice. Ther. Drug Monit. 22:574-581 (2000).
A. Haj-Yehia and M. Bialer. Structure-pharmacokinetic relationships in a series of short fatty acid amides that possess anticonvulsant activity. J. Pharm. Sci. 79:719-724 (1990).
W. C. Brown, D. O. Schiffman, E. A. Swinyard, and L. S. Goodman. Comparative assay of antiepileptic drugs by “psychomotor” seizures and minimal electroshock threshold test. J. Pharmacol. Exp. Ther. 107:273-283 (1953).
M. E. Barton, B. D. Klein, H. H. Wolf, and H. S. White. Pharmacological characterization of the 6Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 47:217-227 (2001).
E. A. Swinyard, J. H. Woodhead, H. S. White, and M. R. Franklin. General principles: experimental selection, quantification and evaluation of antiepileptic drugs. In R. H. Levy, R. H. Mattson, B. S. Meldrum, K. J. Penry, F. E. Dreifuss (eds.), Antiepileptic Drugs, 3rd ed. Raven Press, New York, 1989, pp. 85-102.
H. S. White, J. H. Woodhead, K. S. Wilcox, J. P. Stables, H. J. Kupferberg, and H. H. Wolf. General principles: discovery and preclinical development of antiepileptic drugs. In R. H. Levy, R. H. Mattson, B. S. Melddrum, E. Perucca (eds.), Antiepileptic Drugs, 5th ed. Lippincott Williams and Wilkins, Philadelphia, Pennsylvania, 2002, pp. 36-48.
E. W. Lothman, R. A. Salerno, J. B. Perlin, and D. L. Kaiser. Screening and characterization of antiepileptic drugs with rapidly recurring hippocampal seizures in rats. Epilepsy Res. 2:367-379 (1988).
R. J. Racine. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroenceph. Clin. Neurophysiol. 32:281-294 (1972).
D. J. Finney. Probit Analysis, 3rd ed. Cambridge University Press, Cambridge, United Kingdom, 1971.
V. P. Shah, K. K. Midha, J. W. A. Findlay, H. M. Hill, J. D. Hulse, I. J. McGilveray, G. McKay, K. J. Miller, R. N. Patnaik, M. L. Powell, A. Tonell, C. T. Viswanathan, and A. Yacobi. Bioanalytical method validation—A revisit with a decade of progress. Pharm. Res. 17:1551-1557 (2000).
M. Gibaldi and D. Perrier. Pharmacokinetics, 2nd ed. Marcel Dekker, New York, 1982.
A. J. Bailer. Testing for the equality of area under the curves when using destructive measurement techniques. J. Pharmacokin. Biopharm. 16:303-309 (1988).
J. Yuan. Estimation of variance for AUC in animal studies. J. Pharm. Sci. 82:761-763 (1993).
R. H. Levy and A. V. Boddy. Stereoselectivity in pharmacokinetics: a general theory. Pharm. Res. 8:551-555 (1991).
M. Radatz, K. Ehlers, B. Yagen, M. Bialer, and H. Nau. Valnoctamide, valpromide and valnoctic acid are much less teratogenic in mice than valproic acid. Epilepsy Res. 30:41-48 (1998).
N. Isoherranen, B. Yagen, and M. Bialer. New CNS-active drugs which are second-generation valproic acid: can they lead to the development of a magic bullet? Curr. Opin. Neurol. 16:203-211 (2003).
N. Isoherranen. J. H. Woodhead, H. S. White, and M. Bialer. Anticonvulsant profile of valrocemide (TV1901): A new antiepileptic drug. Epilepsia 42:831-836 (2001).
N. Isoherranen, H. S. White, R. H. Finnell, B. Yagen, J. H. Woodhead, G. D. Bennett, K. S. Wilcox, M. Barton, and M. Bialer. Anticonvulsant profile and teratogenicity of N-methyl-tetramethylcyclopropyl carboxamide: a new antiepileptic drug. Epilepsia 43:115-126 (2002).
N. Isoherranen, B. Yagen, J. H. Woodhead, O. Spiegelstein, S. Blotnik, K. S. Wilcox, R. H. Finnell, G. D. Bennett, H. S. White, and M. Bialer. Characterization of the anticonvulsant profile and enantioselective pharmacokinetics of the chiral valproylamide propylisopropyl acetamide in rodents. Br. J. Pharmacol. 138:602-613 (2003).
M. Bialer, A. Rubinstein, J. Dubrovsky, I. Raz, and O. Abramsky. A comparative pharmacokinetic study of valpromide and valproic acid after intravenous administration in humans. Int. J. Pharm. 23:25-33 (1985).
M. Bialer. Clinical pharmacology of valpromide. Clin. Pharmacokinet. 20:114-122 (1991).
F. Pisani, A. Haj-Yehia, A. Fazio, C. Artesi, G. Oteri, E. Perucca, D. L. Kroetz, R. H. Levy, and M. Bialer. Carbamazepine-valnoctamide interaction in epileptic patients: In vitro/in vivo correlation. Epilepsia 34:954-958 (1993).
S. Blotnik, F. Bergman, and M. Bialer. Disposition of valpromide, valproic acid, and valnoctamide in the brain, liver, plasma, and urine of rats. Drug Metab. Dispos. 24:560-564 (1996).
H. Derendorf and B. Meibohm. Modeling of pharmacokinetic/pharmacodynamic (PK/PD) relationships: concepts and perspectives. Pharm. Res. 16:176-185 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Isoherranen, N., White, H.S., Klein, B.D. et al. Pharmacokinetic-Pharmacodynamic Relationships of (2S,3S)-Valnoctamide and Its Stereoisomer (2R,3S)-Valnoctamide in Rodent Models of Epilepsy. Pharm Res 20, 1293–1301 (2003). https://doi.org/10.1023/A:1025069519218
Issue Date:
DOI: https://doi.org/10.1023/A:1025069519218