Abstract
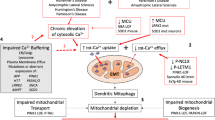
Neuronal damage following stroke or neurodegenerative diseases is thought to stem in part from overexcitation of N-methyl-D-aspartate (NMDA) receptors by glutamate. NMDA receptors triggered neurotoxicity is mediated in large part by activation of neuronal nitric oxide synthase (nNOS) and production of nitric oxide (NO). Simultaneous production of superoxide anion in mitochondria provides a permissive environment for the formation of peroxynitrite (ONOO−). Peroxynitrite damages DNA leading to strand breaks and activation of poly(ADP-ribose) polymerase-1 (PARP-1). This signal cascade plays a key role in NMDA excitotoxicity, and experimental models of stroke and Parkinson's disease. The mechanisms of PARP-1-mediated neuronal death are just being revealed. While decrements in ATP and NAD are readily observed following PARP activation, it is not yet clear whether loss of ATP and NAD contribute to the neuronal death cascade or are simply a biochemical marker for PARP-1 activation. Apoptosis-inducing factor (AIF) is normally localized to mitochondria but following PARP-1 activation, AIF translocates to the nucleus triggering chromatin condensation, DNA fragmentation and nuclear shrinkage. Additionally, phosphatidylserine is exposed and at a later time point cytochrome c is released and caspase-3 is activated. In the setting of excitotoxic neuronal death, AIF toxicity is caspase independent. These observations are consistent with reports of biochemical features of apoptosis in neuronal injury models but modest to no protection by caspase inhibitors. It is likely that AIF is the effector of the morphologic and biochemical events and is the commitment point to neuronal cell death, events that occur prior to caspase activation, thus accounting for the limited effects of caspase inhibitors. There exists significant cross talk between the nucleus and mitochondria, ultimately resulting in neuronal cell death. In exploiting this pathway for the development of new therapeutics, it will be important to block AIF translocation from the mitochondria to the nucleus without impairing important physiological functions of AIF in the mitochondria.
Similar content being viewed by others
REFERENCES
Arnoult, D., Tatischeff, I., Estaquier, J., Girard, M., Sureau, F., Tissier, J. P., Grodet, A., Dellinger, M., Traincard, F., Kahn, A., Ameisen, J. C., and Petit, P. X. (2001). Mol. Biol. Cell 12, 3016–3030.
Berger, N. A., and Berger, S. J. (1986). Basic Life Sci. 38, 357–363.
Berger, N. A., Sims, J. L., Catino, D. M., and Berger, S. J. (1983). Princess Takamatsu Symp. 13, 219–226.
Braun, J. S., Novak, R., Murray, P. J., Eischen, C. M., Susin, S. A., Kroemer, G., Halle, A., Weber, J. R., Tuomanen, E. I., and Cleveland, J. L. (2001). J Infect. Dis. 184, 1300–1309.
Cande, C., Cohen, I., Daugas, E., Ravagnan, L., Larochette, N., Zamzami, N., and Kroemer, G. (2002). Biochimie 84, 215–222.
Chan, P. H. (2001). J. Cereb. Blood Flow Metab. 21,2–14.
Chiarugi, A. (2002). Trends Pharmacol. Sci. 23, 122–129.
Cregan, S. P., Fortin, A., MacLaurin, J. G., Callaghan, S. M., Cecconi, F., Yu, S. W., Dawson, T. M., Dawson, V. L., Park, D. S., Kroemer, G., and Slack, R. S. (2002). J. Cell Biol. 158, 507–517.
Dawson, V. L., and Dawson, T. M. (1998). Prog. Brain. Res. 118, 215–229.
de Murcia, G., and Menissier de Murcia, J. (1994). Trends Biochem. Sci. 19, 172–176.
Dirnagl, U., ladecola, C., and Moskowitz, M. A. (1999). Trends Neurosci. 22, 391–397.
Eliasson, M. J., Sampei, K., Mandir, A. S., Hurn, P. D., Traystman, R. J., Bao, J., Pieper, A., Wang, Z. Q., Dawson, T. M., Snyder, S. H., and Dawson, V. L. (1997). Nat. Med., 3, 1089–1095.
Endres, M., Wang, Z. Q., Namura, S., Waeber, C., and Moskowitz, M. A. (1997). J. Cereb. Blood Flow Metab. 17, 1143–1151.
Goto, S., Xue, R., Sugo, N., Sawada, M., Blizzard, K. K., Poitras, M. F., Johns, D. C., Dawson, T. M., Dawson, V. L., Crain, B. J., Traystman, R. J., Mori, S., and Hurn, P. D. (2002). Stroke 33, 1101–1106.
Hageman, G. J., and Stierum, R. H. (2001). Mutat. Res. 475,45–56.
Hisatomi, T., Sakamoto, T., Goto, Y., Yamanaka, I., Oshima, Y., Hata, Y., Ishibashi, T., Inomata, H., Susin, S. A., and Kroemer, G. (2002). Curr. Eye Res. 24, 161–172.
Ischiropoulos, H., and Beckman, J. S. (2003). J. Clin. Invest. 111, 163–169.
Joza, N., Susin, S. A., Daugas, E., Stanford, W. L., Cho, S. K., Li, C. Y., Sasaki, T., Elia, A. J., Cheng, H. Y., Ravagnan, L., Ferri, K. F., Zamzami, N., Wakeham, A., Hakem, R., Yoshida, H., Kong, Y. Y., Mak, T. W., Zuniga-Pflucker, J. C., Kroemer, G., and Penninger, J. M. (2001). Nature 410, 549–554.
Klein, J. A., Longo-Guess, C. M., Rossmann, M. P., Seburn, K. L., Hurd, R. E., Frankel, W. N., Bronson, R. T., and Ackerman, S. L. (2002). Nature, 419, 367–374.
Kristian, T., and Siesjo, B. K. (1998). Stroke 29, 705–718.
Lassus, P., Opitz-Araya, X., and Lazebnik, Y. (2002). Science, 297, 1352–1354.
Lindahl, T., Satoh, M. S., Poirier, G. G., and Klungland, A. (1995). Trends Biochem. Sci. 20, 405–411.
Lipton, P. (1999). Physiol. Rev. 79, 1431–1568.
Loeffler, M., Daugas, E., Susin, S. A., Zamzami, N., Metivier, D., Nieminen, A. L., Brothers, G., Penninger, J. M., and Kroemer, G. (2001). FASEB J. 15, 758–767.
Mandir, A. S., Pizedborski, S., Jackson-Lewis, V., Wang, Z. Q., Simbulan-Rosenthal, C. M., Smulson, M. E., Hoffman, B. E., Guastella, D. B., Dawson, V. L., and Dawson, T. M. (1999). Proc. Natl. Acad. Sci. USA 96, 5774–5779.
Mate, M. J., Ortiz-Lombardia, M., Boitel, B., Haouz, A., Tello, D., Susin, S. A., Penninger, J., Kroemer, G., and Alzari, P. M. (2002). Nat. Struct. Biol. 9, 442–446.
Mayer, M. L., and Westbrook, G. L. (1987). Prog. Neurobiol. 28, 197–276.
Miramar, M. D., Costantini, P., Ravagnan, L., Saraiva, L. M., Haouzi, D., Brothers, G., Penninger, J. M., Peleato, M. L., Kroemer, G., and Susin, S. A. (2001). J. Biol. Chem., 276, 16391–16398.
Robertson, J. D., Enoksson, M., Suomela, M., Zhivotovsky, B., and Orrenius, S. (2002). J. Biol. Chem., 277, 29808–29809.
Samdani, A. F., Dawson, T. M., and Dawson, V. L. (1997). Stroke 28, 1283–1288.
Susin, S. A., Daugas, E., Ravagnan, L., Samejima, K., Zamzami, N., Loeffler, M., Costantini, P., Ferri, K. F., Irinopoulou, T., Prevost, M. C., Brothers, G., Mak, T. W., Penninger, J., Earnshaw, W. C., and Kroemer, G. (2000). J. Exp. Med. 192, 571–580.
Susin, S. A., Lorenzo, H. K., Zamzami, N., Marzo, I., Snow, B. E., Brothers, G. M., Mangion, J., Jacotot, E., Costantini, P., Loeffler, M., Larochette, N., Goodlett, D. R., Aebersold, R., Siderovski, D. P., Penninger, J. M., and Kroemer, G. (1999). Nature 397, 441–446.
Szabo, C., and Dawson, V. L. (1998). Trends Pharmacol. Sci. 19, 287–298.
Wang, X., Yang, C., Chai, J., Shi, Y., and Xue, D. (2002). Science, 298, 1587–1592.
Ye, H., Cande, C., Stephanou, N. C., Jiang, S., Gurbuxani, S., Larochette, N., Daugas, E., Garrido, C., Kroemer, G., and Wu, H. (2002). Nat. Struct. Biol. 9, 680–684.
Yu, S. W. Wang, H., Poitras, M. F., Coombs, C., Bowers, W. J., Federoff, H. J., Poirier, G. G., Dawson, T. M., and Dawson, V. L. (2002). Science 297, 259–263.
Zamzami, N., El Hamel, C., Maisse, C., Brenner, C., Munoz-Pinedo, C., Belzacq, A. S., Costantini, P., Vieira, H., Loeffler, M., Molle, G., and Kroemer, G. (2000). Oncogene, 19, 6342–6350.
Zhang, J., Dawson, V. L., Dawson, T. M., and Snyder, S. H. (1994). Science 263, 687–689.
Zhang, X., Chen, J., Graham, S. H., Du, L., Kochanek, P. M., Draviam, R., Guo, F., Nathaniel, P. D., Szabo, C., Watkins, S. C., and Clark, R. S. (2002). J. Neurochem. 82, 181–191.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dawson, V.L., Dawson, T.M. Deadly Conversations: Nuclear-Mitochondrial Cross-Talk. J Bioenerg Biomembr 36, 287–294 (2004). https://doi.org/10.1023/B:JOBB.0000041755.22613.8d
Issue Date:
DOI: https://doi.org/10.1023/B:JOBB.0000041755.22613.8d