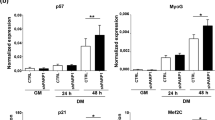
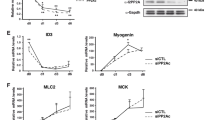
Abstract
Members of the myocyte enhancer factor-2 (MEF2) family of transcription factors associate with myogenic basic helix–loop–helix transcription factors such as MyoD to activate skeletal myogenesis1. MEF2 proteins also interact with the class II histone deacetylases HDAC4 and HDAC5, resulting in repression of MEF2-dependent genes2,3,4. Execution of the muscle differentiation program requires release of MEF2 from repression by HDACs, which are expressed constitutively in myoblasts and myotubes5. Here we show that HDAC5 shuttles from the nucleus to the cytoplasm when myoblasts are triggered to differentiate. Calcium/calmodulin-dependent protein kinase (CaMK) signalling, which stimulates myogenesis5 and prevents formation of MEF2–HDAC complexes4, also induces nuclear export of HDAC4 and HDAC5 by phosphorylation of these transcriptional repressors. An HDAC5 mutant lacking two CaMK phosphorylation sites is resistant to CaMK-mediated nuclear export and acts as a dominant inhibitor of skeletal myogenesis, whereas a cytoplasmic HDAC5 mutant is unable to block efficiently the muscle differentiation program. Our results highlight a mechanism for transcriptional regulation through signal- and differentiation-dependent nuclear export of a chromatin-remodelling enzyme, and suggest that nucleo-cytoplasmic trafficking of HDACs is involved in the control of cellular differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Molkentin, J. D., Black, B. L., Martin, J. F. & Olson, E. N. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell 83, 1125– 1136 (1995).
Sparrow, D. B. et al. MEF-2 function is modified by a novel co-repressor, MITR. EMBO J. 18, 5085–5098 (1999).
Miska, E. A. et al. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J. 18, 5099– 5107 (1999).
Lu, J., McKinsey, T. A., Nicol, R. L. & Olson, E. N. Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc Natl Acad Sci. USA 97, 4070–4075 (2000).
Lu, J., McKinsey, T. A., Zhang, C. L. & Olson, E. N. Regulation of skeletal myogenesis by association of MEF2 with class II histone deacetylases. Mol. Cell 6, 233– 244 (2000).
Kuo, M. H. & Allis, C. D. Roles of histone acetyltransferases and deacetylases in gene regulation. BioEssays 20, 615–626 (1998).
Han, J., Jiang, Y., Li, Z., Kravchenko, V. V. & Ulevitch, R. J. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature 386, 296–299 (1997).
Kato, Y. et al. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 16, 7054–7066 (1997).
Mao, Z. & Wiedmann, M. Calcineurin enhances MEF2 DNA binding activity in calcium-dependent survival of cerebellar granule neurons. J. Biol. Chem. 274, 31102–31107 (1999).
Wu, H. et al. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 19, 1–11 (2000).
Nishi, K. et al. Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J. Biol. Chem. 269, 6320–6324 (1994).
Fukuda, M. et al. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390, 308 –311 (1997).
Gorner, W. et al. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 12, 586–597 ( 1998).
Beals, C. R., Sheridan, C. M., Turck, C. W., Gardner, P. & Crabtree, G. R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275, 1930–1934 (1997).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999).
Pinna, L. A. & Ruzzene, M. How do protein kinases recognize their substrates? Biochem. Biophys. Acta. 1314, 191–225 (1996).
Grozinger, C. M. & Schreiber, S. L. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc. Natl Acad. Sci. USA 97 , 7835–7840 (2000).
Sartorelli, V., Huang, J., Hamamori, Y. & Kedes, L. Molecular mechanisms of myogenic coactivation by p300: direct interaction with the activation domain of MyoD and with the MADS box of MEF2C. Mol. Cell. Biol. 17, 1010–1026 (1997).
Mayford, M. et al. Control of memory formation through regulated expression of a CaMKII transgene. Science 274, 1678– 1683 (1996).
Passier, R. et al. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J. Clin. Invest. 105, 1395–1406 (2000).
Black, B. L. & Olson, E. N. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins.. Annu. Rev. Cell Dev. Biol. 14, 167–196 (1998).
Grozinger, C. M., Hassig, C. A. & Schreiber, S. L. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl. Acad. Sci. 96, 4868–4873 (1999).
Haribabu, B. et al. Human calcium-calmodulin dependent protein kinase I: cDNA cloning, domain structure and activation by phosphorylation at threonine-177 by calcium-calmodulin dependent protein kinase I kinase. EMBO J. 14, 3679–3686 ( 1995).
Chatila, T., Anderson, K. A., Ho, N. & Means, A. R. A unique phosphorylation-dependent mechanism for the activation of Ca2+/calmodulin-dependent protein kinase type IV/GR. J. Biol. Chem. 271, 21542– 21548 (1996).
O'Keefe, S. J., Tamura, J., Kincaid, R. L., Tocci, M. J. & O'Neill, E. A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature 357, 692–694 (1992).
Jiang, Y. et al. Characterization of the structure and function of a new mitogen-activated protein kinase (p38beta). J. Biol. Chem. 271, 17920–17926 (1996).
Rybkin, I. I., Cross, M. E., McReynolds, E. M., Lin, R. Z. & Ballou, L. M. alpha(1A) adrenergic receptor induces eukaryotic initiation factor 4E-binding protein 1 phosphorylation via a Ca(2+)-dependent pathway independent of phosphatidylinositol 3-kinase/Akt. J. Biol. Chem. 275, 5460– 5465 (2000).
English, J. M. et al. Contribution of the ERK5/MEK5 pathway to Ras/Raf signaling and growth control. J. Biol. Chem. 274, 31588–31592 (1999).
Stambolic, V. & Woodgett, J. R. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem. J. 303, 701–704 (1994).
Mellon, P. L., Clegg, C. H., Correll, L. A. & McKnight, G. S. Regulation of transcription by cyclic AMP-dependent protein kinase. Proc. Natl Acad. Sci. USA 86, 4887– 4891 (1989).
Acknowledgements
We thank M. Cobb, J. Han, R. Lin, G.S. McKnight, A. Means, S. O'Keefe, S. Schreiber, T. Chatila and J. Woodgett for expression plasmids; R. Prives for anti-MEF2 antisera; and M. Yoshida for leptomycin B. We are grateful to A. Tizenor for graphics, and J. Page and W. Simpson for editorial assistance. E.N.O. was supported by grants from NIH, the Robert A. Welch Foundation, the D. W. Reynolds Foundation and Myogen, Inc. T.A.M. is a Pfizer fellow of The Life Sciences Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McKinsey, T., Zhang, CL., Lu, J. et al. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408, 106–111 (2000). https://doi.org/10.1038/35040593
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35040593
This article is cited by
-
Deciphering the roles of subcellular distribution and interactions involving the MEF2 binding region, the ankyrin repeat binding motif and the catalytic site of HDAC4 in Drosophila neuronal morphogenesis
BMC Biology (2024)
-
Progress on the roles of MEF2C in neuropsychiatric diseases
Molecular Brain (2022)
-
The role of histone modifications: from neurodevelopment to neurodiseases
Signal Transduction and Targeted Therapy (2022)
-
Modulation of cellular processes by histone and non-histone protein acetylation
Nature Reviews Molecular Cell Biology (2022)
-
Paeoniflorin ameliorates ischemic injury in rat brain via inhibiting cytochrome c/caspase3/HDAC4 pathway
Acta Pharmacologica Sinica (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.