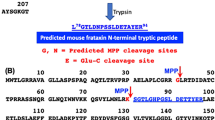
Abstract
Friedreich ataxia (FRDA), the most common autosomal recessive ataxia, is characterized by degeneration of the large sensory neurons and spinocerebellar tracts, cardiomyopathy and increased incidence in diabetes1,2. FRDA is caused by severely reduced levels of frataxin, a mitochondrial protein3 of unknown function. Yeast knockout models as well as histological and biochemical data from heart biopsies or autopsies of FRDA patients have shown that frataxin defects cause a specific iron-sulfur protein deficiency and intramitochondrial iron accumulation4,5,6,7. We have recently shown that complete absence of frataxin in the mouse leads to early embryonic lethality8, demonstrating an important role for frataxin during mouse development. Through a conditional gene-targeting approach, we have generated in parallel a striated muscle frataxin-deficient line and a neuron/cardiac muscle frataxin-deficient line, which together reproduce important progressive pathophysiological and biochemical features of the human disease: cardiac hypertrophy without skeletal muscle involvement, large sensory neuron dysfunction without alteration of the small sensory and motor neurons, and deficient activities of complexes I–III of the respiratory chain and of the aconitases. Our models demonstrate time-dependent intramitochondrial iron accumulation in a frataxin-deficient mammal, which occurs after onset of the pathology and after inactivation of the Fe-S-dependent enzymes. These mutant mice represent the first mammalian models to evaluate treatment strategies for the human disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Durr, A. et al. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N. Engl. J. Med. 335, 1169–1175 (1996).
Harding, A.E. Friedreich's ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain 104, 589–520 (1981).
Campuzano, V. et al. Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum. Mol. Genet. 6, 1771–1780 (1997).
Babcock, M. et al. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science 276, 1709–1712 (1997).
Foury, F. & Cazzalini, O. Deletion of the yeast homologue of the human gene associated with Friedreich's ataxia elicits iron accumulation in mitochondria. FEBS Lett. 411, 373–377 (1997).
Rotig, A. et al. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nature Genet. 17, 215–217 (1997).
Lamarche, J.B., Shapcott, D., Cote, M. & Lemieux, B. Cardiac iron deposits in Friedreich's ataxia. in Handbook of Cerebellar Diseases (ed. Lechtenberg, R.) 453–458 (Marcel Dekker, New York, 1993).
Cossee, M. et al. Inactivation of the Friedreich ataxia mouse gene leads to early embryonic lethality without iron accumulation. Hum. Mol. Genet. 9, 1219–1226 (2000).
Wang, J. et al. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nature Genet. 21, 133–137 (1999).
Frugier, T. et al. Nuclear targeting defect of SMN lacking the C-terminus in a mouse model of spinal muscular atrophy. Hum. Mol. Genet. 9, 849–858 (2000).
Watmough, N.J. et al. Impaired mitochondrial β-oxidation in a patient with an abnormality of the respiratory chain. Studies in skeletal muscle mitochondria. J. Clin. Invest. 85, 177–184 (1990).
Radisky, D.C., Babcock, M.C. & Kaplan, J. The yeast frataxin homologue mediates mitochondrial iron efflux. Evidence for a mitochondrial iron cycle. J. Biol. Chem. 274, 4497–4499 (1999).
Adamec, J. et al. Iron-dependent self-assembly of recombinant yeast frataxin: implications for Friedreich ataxia. Am. J. Hum. Genet. 67, 549–562 (2000).
Musco, G. et al. Towards a structural understanding of Friedreich's ataxia: the solution structure of frataxin. Structure Fold Des. 8, 695–707 (2000).
Dhe-Paganon, S., Shigeta, R., Chi, Y.I., Ristow, M. & Shoelson, S.E. Crystal structure of human frataxin. J. Biol. Chem. 275, 30753–30756 (2000).
Foury, F. Low iron concentration and aconitase deficiency in a yeast frataxin homologue deficient strain. FEBS Lett. 456, 281–284 (1999).
Campuzano, V. et al. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271, 1423–1427 (1996).
Koutnikova, H. et al. Studies of human, mouse and yeast homologues indicate a mitochondrial function for frataxin. Nature Genet. 16, 345–351 (1997).
Jiralerspong, S., Liu, Y., Montermini, L., Stifani, S. & Pandolfo, M. Frataxin shows developmentally regulated tissue-specific expression in the mouse embryo. Neurobiol. Dis. 4, 103–113 (1997).
Dierich, A. & Dollé, P. in Methods in Developmental Toxicology and Biology (ed. Klug, S.a.T.) 111–123 (Blackwekk Science, Oxford, 1997).
Sambrook, J., Fritsch, E.F. & Maniatis, T. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 1989).
Rustin, P. et al. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta 228, 35–51 (1994).
Dupe, V. et al. In vivo functional analysis of the Hoxa-1 3′ retinoic acid response element (3′RARE). Development 124, 399–410 (1997).
Doevendans, P.A., Daemen, M.J., de Muinck, E.D. & Smits, J.F. Cardiovascular phenotyping in mice. Cardiovasc. Res. 39, 34–49 (1998).
Burck, U., Goebel, H.H., Kuhlendahl, H.D., Meier, C. & Goebel, K.M. Neuromyopathy and vitamin E deficiency in man. Neuropediatrics 12, 267–278 (1981).
Koenig, M. Friedreich ataxia and AVED. in The Metabolic and Molecular Basis of Inherited Disease (eds. Scriver, C, Beaudet, A, Sly, W, & Valle, D.) 5845–5855 (MacGraw-Hill, New York, 2001).
Acknowledgements
We thank J.L. Mandel and members of his laboratory for discussions and comments; L. Reutenauer, N. Lagarde and P. Goetz-Reiner for technical support; N. Calizot for discussions and comments during the EMG analysis; and R. Kahn for the MCK-Cre transgenic animals. This work was supported by funds from the Human Frontier Science Program (HFSP), the European Community (contract QLRT-CT-1999-00584), the Association Française contre les Myopathies (AFM), the Association Française contre l'ataxie de Friedreich (AFAF), the Muscular Dystrophy Association of America (to M.K.), INSERM, CNRS and the Hôpital Universitaire de Strasbourg (HUS). M.C. is supported by the INSERM, H.P. by the HFSP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puccio, H., Simon, D., Cossée, M. et al. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat Genet 27, 181–186 (2001). https://doi.org/10.1038/84818
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/84818
This article is cited by
-
Human frataxin, the Friedreich ataxia deficient protein, interacts with mitochondrial respiratory chain
Cell Death & Disease (2023)
-
Cerebellum Lecture: the Cerebellar Nuclei—Core of the Cerebellum
The Cerebellum (2023)
-
AAV-vector based gene therapy for mitochondrial disease: progress and future perspectives
Orphanet Journal of Rare Diseases (2022)
-
Cur@SF NPs alleviate Friedreich’s ataxia in a mouse model through synergistic iron chelation and antioxidation
Journal of Nanobiotechnology (2022)
-
Mice harboring the FXN I151F pathological point mutation present decreased frataxin levels, a Friedreich ataxia-like phenotype, and mitochondrial alterations
Cellular and Molecular Life Sciences (2022)