Abstract
Ion channel hyperactivation can result in neuronal loss in injury, stroke and neurodegenerative disease. Acidosis-associated hyperactivation of the Degenerin/epithelial amiloride-sensitive Na+ channel (DEG/ENaC) acid-sensing ion channel 1a (ASIC1a), a proton-gated channel expressed in the mammalian brain, contributes significantly to neuronal loss in ischemia. Analogously, in invertebrates, genetic hyperactivation of the Caenorhabditis elegans mechanosensory (MEC) channel (MEC-4(d)) of the DEG/ENaC ion channel superfamily induces neuronal necrosis. Similarly substituted MEC-10(d) mutant subunits of the same MEC channel are only marginally neurotoxic, and we therefore exploited the weak necrosis phenotype of mec-10(d) lines to screen for novel extragenic mutations that enhance neuronal death. Here, we report on one mec-10(d) necrosis enhancer, which we show is MEC-4 variant MEC-4(A149V). MEC-4(A149V) executes normal MEC-4 function in touch sensation and does not induce necrosis on its own, but rather combines with MEC-10(d) to create a strongly neurotoxic channel. The MEC-4(A149V)+MEC-10(d) channel conducts elevated Na+ and Ca2+ currents (with a disproportionate increase in Ca2+ current) in the Xenopus oocyte expression system, and exhibits altered binding of the channel inhibitor amiloride. Our data document the first example of synergistically toxic intersubunit interactions in the DEG/ENaC channel class and provide evidence that Ca2+ current levels may be decisive factors in tipping the balance between neuronal survival and necrosis.
Similar content being viewed by others
Main
Ion channel dysfunction can result in the neuronal death that underlies the devastating consequences of stroke and nervous system injury. Primary determinants of mammalian neuronal loss associated with ion channel hyperactivation include the glutamate-gated channels1 and the less extensively studied ASIC (acid-sensing ion channels)2 of the DEG/ENaC superfamily (named after founding members Caenorhabditis elegans degenerins and the mammalian epithelial amiloride-sensitive Na+ channels). DEG/ENaC channel complexes include three DEG/ENaC subunits,3 with each subunit having a large extracellular domain, two transmembrane domains that contribute to the channel pore, and intracellular N and C termini (reviewed in Kellenberger and Schild4). Structure/activity studies in the DEG/ENaC channel class have provided insights into function, but the relationship between toxicity and subunit interactions is largely unexplored.
Of DEG/ENaCs studied to date, the C. elegans MEC channel, which transduces gentle touch stimuli in six mechanosensory (MEC) neurons5, 6 has been analyzed in most genetic detail. The core of the MEC channel is formed by DEG/ENaC subunits MEC-47, 8 and MEC-10,9 which associate with paraoxonase-like transmembrane protein MEC-610 and stomatin-like protein MEC-2.7, 11, 12 Dominant gain-of-function mutations alter MEC-4(A713) (the d position) to introduce large amino acids (AAs) near the channel pore, causing channel hyperactivity that induces necrotic-like neurodegeneration.8, 11, 13 Analogous substitutions for MEC-10 (MEC-10(d), position A673) are only weakly neurotoxic.9 Neurotoxic MEC-4(d) channels conduct both elevated Na+ and Ca2+ currents13 and exhibit commonalities with the hyperactivation of the Na+- and Ca2+-permeable mammalian ASIC1a channel,14 which, when overstimulated by brain acidosis in ischemia, causes neurodegeneration.2 Understanding mechanisms by which neuronally expressed DEG/ENaCs are hyperactivated to become toxic is thus of considerable significance in pathobiology.
To elaborate molecular mechanisms of channel neurotoxicity within a physiological context, we screened for new extragenic mutations that enhance the weak mec-10(d)-induced neurodegeneration. Here, we report on death enhancer allele bz301, which we show is a novel mec-4 allele encoding MEC-4(A149V) substitution in the channel extracellular domain. MEC-4(A149V) functions normally in touch sensation and induces neurodegeneration only in combination with the MEC-10(d) subunit. Our electrophysiological analysis supports that the MEC-4(A149V)+MEC-10(d) channel is hyperactivated and preferentially calcium permeable, with altered amiloride binding properties. These data inform on quaternary channel relationships and provide an example of intersubunit interaction toxicity that suggests that allelic variation within the mammalian ASIC channel class might influence susceptibility to ischemia.
Results
A screen for enhancers of mec-10(d)-induced neurodegeneration
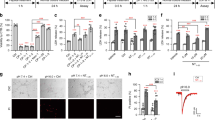
We constructed a strain that expresses both transgenically introduced mec-10(d)9 and a green fluorescent protein (GFP) reporter (to highlight touch neurons) in the touch neurons (we named this strain Ismec-10(d)). We evaluated survival of touch neurons in Ismec-10(d) at the larval stage 4 (L4)/young adult stage by scoring the numbers of fluorescent posterior lateral microtubule neurons (PLM) in the tail, which are positioned away from the gut autofluorescence and are, thus, unambiguously identified (Figure 1a). In nematodes reared at 20°C, most PLM neurons in Ismec-10(d) are viable despite the expression of weak necrosis inducer mec-10(d). Ismec-10(d) does exhibit some necrosis at 15°C, confirming the potential for necrosis induction by the mec-10(d) transgene array (Figure 1a). We mutagenized parental line Ismec-10(d) by ethyl methyl sulfonate (EMS) treatment, and screened 18 500 genomes for novel mutants lacking some or all of the fluorescent touch neurons, revealing enhanced neuronal loss (Figure 1b and c). We identified 18 mutants that exhibit strong/intermediate enhancement of necrosis, as well as several weaker death enhancers. Here, we describe molecular identification and characterization of bz301, one of the strong necrosis enhancers (Figure 1c, right panel).
A green fluorescent protein (GFP)-based screen for the enhancers of neurodegeneration induced by transgene mec-10(d). (a) Quantitation of touch cell loss in parental strain Ismec-10(d). mec-10(d) induces marginal necrosis in touch neurons at 20°C, but is more toxic at 15°C. The X axis indicates how many of the two PLM neurons (0, 1, or 2) can be detected in wild type (WT; black bar) and in Ismec-10(d) at 20°C (gray) or at 15°C (white). n=700 (in seven independent trials) at 20°C and n=400 (in four independent trials) at 15°C; **P<0.01 by comparison with WT, by t-test. These data establish that Ismec-10(d) has the potential to induce necrosis, but exhibits a low baseline of cell death at 20°C. (b) Strategy for identifying enhancers of mec-10(d)-induced neurodegeneration. In the parental strain Ismec-10(d) (which is zdIs5[pmec-4GFP] I; bzIs67[mec-10(d)] X), there is actually little necrosis, and thus the six touch receptor neurons almost always survive and fluoresce. Mutants that harbor a new necrosis enhancer mutation consequent to ethyl methyl sulfonate (EMS) treatment will have less GFP signal due to touch receptor neuron death. (c) An example of touch receptor fluorescence in the parental strain (left panel) and in the bz301 enhancer mutant background (right panel). In the bz301 strain, four touch neurons have died and only AVM and PVM survive
bz301 is a semi-dominant enhancer of Ismec-10(d) that exacerbates necrosis in a calreticulin-dependent mechanism
In the Ismec-10(d) bz301 mutant (20°C), 49% of young L1 larvae have one to two necrotic PLMs (Figure 2a), and yet 84% lack one to two PLMs by the L4 stage (Figure 2b). The fact that more neurons are dead by the L4 stage than appear dying at the L1 stage suggests that necrosis onset can be after the L1 stage in the Ismec-10(d) bz301 strain, and we confirmed this by visual inspection of L2 and L3 larvae that were devoid of swollen necrotic neurons as L1s (data not shown). Although bz301 is a strong necrosis enhancer, its effects are less potent than observed for mec-4(d) – in mec-4(d) mutants, 95% of L1 larvae have one to two necrotic PLM neurons and 100% have either 1 or 2 neurons dead by the L4 stage. We find a significant amount of death in the bz301/+ heterozygotes, which approaches that in bz301/bz301 homozygotes (Figure 2c). These results establish the genetically semi-dominant action of allele bz301 in necrosis enhancement.
bz301 acts semi-dominantly to enhance neuronal loss in Ismec-10(d) by a calreticulin-dependent mechanism. (a) Quantitation of swollen necrotic PLM touch neurons during the early L1 stage (within 4 h after hatching) in Ismec-10(d) (black), Ismec-10(d) bz301 (dark gray), crt-1(bz29); Ismec-10(d) bz301 (light gray), and mec-4(d) (white) animals; n⩾230 in at least three independent trails, at 20 °C. (b) Quantitation of surviving fluorescent PLM touch neurons at the L4 stage for Ismec-10(d) (black), Ismec-10(d) bz301 (dark gray), crt-1(bz29); Ismec-10(d) bz301 (light gray), and mec-4(d) (white) animals. A comparison of the extent of PLM swelling and PLM death for Ismec-10(d) bz301 reveals that more PLMs die than appear as swollen necrotic figures in the L1 stage, suggesting that neurodegeneration can have late larval onset, and we confirmed this by visual inspection of older larvae (data not shown); n⩾170 in at least three independent trails, at 20°C. The crt-1 null mutation suppresses cell death induced in Ismec-10(d) bz301. (c) bz301 acts semi-dominantly. We counted the number of surviving touch receptor neurons (of six total) in bz301 homozygotes (black) or bz301 heterozygotes (gray). Ismec-10(d) is homozygous in all lines. bz301 heterozygotes and homozygotes have extensive neuronal loss, but nearly all Ismec-10(d) homozygotes (white) have six touch cells surviving
We also tested whether enhancer bz301 activates necrosis with similar genetic requirements to mec-4(d). In mec-4(d)-induced death, progression through necrosis requires calreticulin, a calcium-storing ER (endoplasmic reticulum) chaperone, which we propose is needed for the release of ER calcium stores and amplification of toxic Ca2+ overload.15 We constructed the triple mutant crt-1; Ismec-10(d) bz301 to ask whether the enhanced death is blocked by the crt-1 null mutation. We found that calreticulin deficiency fully blocks necrosis-enhancing effects of bz301 (Figure 2a and b). We concluded that bz301-induced necrosis involves a mechanism similar to that induced by the MEC-4(d) channel.
Necrosis enhancer bz301 encodes MEC-4(A149V), adjacent to a conserved extracellular domain
One class of necrosis enhancer that could have been identified in our screen could include mec-4 mutations that themselves induce necrosis.8, 16 As our genetic mapping placed bz301 on the X chromosome near mec-4, we sequenced the mec-4 coding sequence in this mutant background. We found that bz301 does encode a mec-4 mutation, distinct from previously sequenced mec-4 alleles,17, 18 specifying AA change A149V (Figure 3). A149 is located 19 AAs from MEC-4 membrane-spanning domain I (MSDI) on the extracellular side of the protein, adjacent to a conserved DEG/ENaC domain of unknown function (Figure 3a and b). This residue is commonly a nonpolar residue or an Ala (in 32 out of 40 family members; Figure 3b and Supplementary Figure 1), and 4 DEG/ENaC family members (snail FaNaC, C. elegans DEG-1, and two uncharacterized C. elegans family members) normally encode Val at this position (Supplementary Figure 1). Interestingly, this region was found to be a site of interaction between two adjacent subunits within the trimeric channel complex in the recently solved ASIC1a structure.3
bz301 is a mec-4 allele that encodes A149V substitution adjacent to a highly conserved extracellular domain. (a) Cartoon representing the transmembrane topology of the MEC-4 subunit and the position of the A149V substitution. Relatively, short N- and C-terminal MEC-4 domains project into the cell and a single large central loop containing three conserved cysteine-rich domains (CRDI, II, and III) extends extracellularly. The A149 position is indicated by a light gray dot and an arrow. The highly conserved region right after A149 is indicated in dark gray. A short loop preceding membrane-spanning domain II (MSDII) is believed to participate in pore formation (gray) and MSDII contributes to the channel pore. The highly conserved d position MEC-4(A713) at which large side chain amino acid (AA) substitution hyperactivates the channel is indicated by a black dot. Not all domains are drawn to scale. (b) AA sequence alignment of MEC-4 and several DEG/ENaC family members in the region corresponding to MEC-4(A149). The AA change specified by mec-4 allele bz301 is noted at the position indicated by an arrow and a box, AA numbers correspond to MEC-4 primary sequence, position of membrane-spanning domain I is indicated. Included in the alignment are some better-studied C. elegans family members, human ASICs and ENaCs, and fly PPK-1 and RPK-1. Residues common to all DEG/ENaCs are boxed in black; similar residues are boxed in gray. (c) Confirmation that bz301 is the causative mutation for neurodegeneration. The necrosis-enhancer property is also observed for an engineered transgene bzEx170[pmec-4(bz301)] (gray) introduced into the Ismec-10(d) background but not bzEx177[pmec-4(+)] (black), supporting that the necrosis-enhancer property is conferred by the bz301 mutation
To confirm that the bz301-encoded mec-4 mutation is causative for necrosis enhancement, we constructed an mec-4(bz301) allele by site-directed mutagenesis and introduced the pmec-4(bz301) allele into the Ismec-10(d) background (Figure 3c). We find that pmec-4(bz301) induces necrosis. We conclude that the MEC-4(A149V) change is responsible for necrosis-enhancer activity.
mec-4(bz301) requires Ismec-10(d), but not mec-10(+), to induce necrosis
mec-4(bz301) might potentiate neurotoxicity of Ismec-10(d) or, alternatively, mec-4(bz301) could encode a novel mutation that causes necrosis on its own. To distinguish between these two possibilities, we crossed mec-4(bz301) away from Ismec-10(d) and we scored for touch neuron viability in the mec-4(bz301)-only background. We found that mec-4(bz301) does not confer neurotoxicity when present in an otherwise wild-type (WT) background (Figure 4a). Importantly, when we reintroduced a mec-10(d) integrated transgene array different from the one used to generate the parental Ismec-10(d) strain into the bz(301) strain, we found that the neurodegeneration phenotype was restored (Figure 4a). These experiments demonstrate that mec-4(bz301) must act together with mec-10(d) to enhance necrosis and that the neurodegeneration phenotype does not depend on any unusual features of the mec-10(d) transgene array that might have been introduced into the original enhancer mutant background by mutagenesis.
mec-4(bz301) encodes a functional MEC-4 subunit that requires Ismec-10(d) for neurotoxicity. (a) mec-4(bz301) is neurotoxic only in conjunction with Ismec-10(d). bzIs67 X and bzIs75 IV are two independently isolated integrated arrays of mec-10(d), and both are neurotoxic when combined with mec-4(bz301). Y axis indicates the percentage of PLM neurons that undergo degeneration by the L4 stage; n⩾170 in at least three independent trials, at 20°C. All lines have the GFP transgene labeling the six touch neurons (data not shown). MEC-4(A149V) needs MEC-10(d) for its necrotic effect, but cannot induce cell death on its own. (b) The MEC-4(A149V) subunit is a functional MEC-4 subunit. We assayed touch sensitivity of WT (black), mec-4(bz301) (dark gray), bzIs67[mec-10(d)] (light gray), and mec-4(bz301) bzIs67[mec-10(d)] (white) at the L4 stage. We touched animals 10 times and recorded the number of avoidance responses for each animal, n=30, three independent trials performed, at 20°C. As mec-4(bz301) exhibits normal touch sensitivity, the MEC-4(A149V) subunit is functional in vivo. Note that the combinatorial touch insensitivity of mec-4(bz301) and mec-10(d) is consistent with their combinatorial degeneration action
In the combinatorial toxicity situation we characterize for mec-4(bz301)+mec-10(d), the strains we initially tested for neurodegeneration also have two WT genomic copies of mec-10. To address whether mec-10(+) activity is required for synthetic neurotoxicity, we replaced the genomic mec-10(+) copies with mec-10 null deletion allele tm1552, while leaving the mec-10(d) gene array in place. We found that eliminating mec-10(+) alters neither Ismec-10(d)- nor Ismec-10(d);mec-4(bz301)-mediated neuronal degeneration (Supplementary Figure 2A). We conclude that synthetic neurotoxicity requires only mutant MEC-4(A149V) and MEC-10(d) subunits, presumably as components of a hyperactivated heteromeric DEG/ENaC channel.
MEC-4(A149V) functions normally in touch sensation
mec-4(+) is required for sensitivity to gentle touch8 and contributes to the pore of the mechanotransducing complex.19 We wondered whether the MEC-4(A149V) mutant subunit, which does not kill touch neurons on its own, might still possess functional MEC-4 activity. We compared touch sensitivity of WT and mec-4(bz301) mutants to show that touch responses are, in fact, normal in the mec-4(bz301) mutant (Figure 4b, dark gray bar). In the Ismec-10(d);mec-4(bz301) double mutant, the touch response is impaired (Figure 4b, white bar), most likely the consequence of touch receptor degeneration (Ismec-10(d) does not disrupt function on its own (Supplementary Figure 2B). Thus, MEC-4(A149V) is not only non-neurotoxic on its own but also serves as a functional MEC-4 channel subunit.
Overall, we conclude that mec-4(bz301) encodes MEC-4 AA change A149V, which is neither neurotoxic nor channel-disrupting on its own in vivo, but can combine with the Ismec-10(d)-encoded MEC-10(A673V) mutant subunit to generate a strongly neurotoxic channel.
MEC-4(A149V) increases Na+ currents of MEC homomeric and heteromeric channel complexes
The C. elegans MEC channel MEC-4(d)+MEC-10(d)+MEC-2+MEC-6 has been electrophysiologically characterized in Xenopus oocytes10, 11, 13, 20 (see also Figure 5a). In brief, this work showed that (1) channels including the MEC-4(d) subunit conduct markedly elevated Na+ and Ca2+ currents relative to MEC-4(+) channels (note that MEC-4(+) conducts only barely detectable currents); (2) MEC-10(+) and MEC-10(d) do not conduct current as homomeric channels; (3) MEC-10 is not essential for MEC-4(d) channel conductance and dampens MEC channel currents when present; and (4) stomatin MEC-2 and paraoxonase-related MEC-6 subunits increase current.
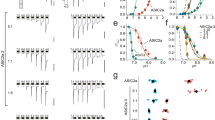
MEC-4(A149V) death-enhancer channel subunits increase currents through MEC-10(d) channels in Xenopus oocytes. (a) An example of sodium currents elicited by voltage steps from −160 to +60 mV from a holding potential of −30 mV in an oocyte injected with mec-4(A149V), mec-10(d), and mec-2 and mec-6 and exposed to a NaCl solution. (b) The average Na+ current at −160 mV recorded from oocytes injected with the subunit compositions indicated on the X axis. In all injections, MEC-2 and MEC-6 cRNA were added to the MEC-4 and/or MEC-10 channel subunit cRNAs. n was 5–21. Data are expressed as mean±SE. **P<0.01 by comparison with all the other channel compositions, by t-test
To gain insight onto the mechanism by which MEC-4(A149V) impacts channel function and promotes neurodegeneration in conjunction with MEC-10(d), we asked how this mutant subunit alters MEC channel properties in the oocyte expression system. We tested combinations of MEC-4 and MEC-10 variant subunits in experiments that included MEC-2 and MEC-6 (Figure 5a and b). Consistent with previous findings, neither MEC-4(+) homomeric channels nor MEC-10(d) homomeric channels conduct significant Na+ current. Similarly, the heteromeric MEC-4(+)+MEC-10(d) channel conducts only marginal Na+ current. By contrast, the MEC-4(d)+MEC-10(d) channel is dramatically hyperactivated, conducting about 26 μA of Na+ current.
How do channels harboring the MEC-4(A149V) subunit compare? In control experiments, we confirmed that MEC-2 and MEC-6 influence the MEC-4(A149V) channel similarly to their influence on the MEC-4(d) channel (Supplementary Figure 3A), indicating that basic subunit interactions within the MEC complex appear maintained. We do note three distinctive differences associated with the expression of MEC-4(A149V) subunits. The first difference is that the homomeric MEC-4(A149V) channel is modestly hyperactivated, conducting currents that are still small (1.2 μA), but are, nonetheless, ∼10 times larger than those of the MEC-4(+) homomeric channel (Figure 5b and Goodman et al.11). This finding suggests that the MEC-4(A149V) substitution might alter homomeric channel open probability or pore conductance properties. We note that if a similar increase in overall channel conductance is associated with a native homomeric MEC-4(A149V) channel in vivo (in the mec-10(+) background), the levels of elevated ion conductance must not reach the threshold for neurotoxicity, because the mec-10(+);mec-4(bz301) double mutant does not exhibit neurodegeneration (Supplementary Figure 2A).
Consistent with the previously reported current-suppressing effect of MEC-10(+) on MEC-4(d) currents,11, 13, 20 we find that the coexpression of MEC-4(A149V) with MEC-10(+) is associated with currents that are smaller relative to MEC-4(A149V) alone. If the native MEC-4(A149V)+MEC-10(+) channel has altered electrophysiological properties in vivo, changes are not substantial enough to disrupt the function in touch sensation, as the mec-4(bz301) mutant is touch sensitive and mec-4 function is required for this behavior (Figure 4b).
The second major difference associated with the MEC-4(A149V) channel involves the heteromeric MEC-4(A149V)+MEC-10(d) combination, which we have shown is toxic in vivo. We find that the current of the MEC-4(A149V)+MEC-10(d) channel is markedly elevated relative to the MEC-4(+)+MEC-10(d) channel, exhibiting ∼15 times higher Na+ currents. This current elevation demonstrates a synergistic activation in the heteromeric MEC-4(A149V)+MEC-10(d) channel that could explain the observed combinatorial toxicity in vivo. The third major difference is the change in amiloride binding of the MEC-4(A149V)+MEC-10(d) heteromeric channel (Supplementary Figure 4), suggesting that both MEC-4 and MEC-10 DEG/ENaC subunits participate in forming the high-affinity amiloride binding site in the channel pore.11, 13
We conclude the MEC-4(A149V)+MEC-10(d) channel is hyperactivated, although to a lesser extent than the MEC-4(d)+MEC-10(d) channel. Interestingly, the conductance levels of the heterologously expressed MEC-4+MEC-10(d) channels parallel the severity of toxicity seen in vivo – the MEC-4(A149V)+MEC-10(d) channel exhibits later onset neurodegeneration and is less toxic than the MEC-4(d)+MEC-10(d) channel in vivo (Figure 2a and b). Like the MEC-4(d) channel, the MEC-4(A149V) channel induces death of Xenopus oocytes (Supplementary Figure 3B).
In summary, the MEC-4(A149V) substitution alone can change MEC channel complex Na+ conductance properties modestly, but in conjunction with MEC-10(d), Na+ currents are significantly increased.
MEC-4(A149V) increases Ca2+ currents of MEC homo- and hetero-multimeric channel complexes
In our previous studies, we found that elevated Ca2+ permeability correlated with MEC-4(d) channel neurotoxicity,13 and therefore we evaluated the Ca2+ conduction of MEC-4(A149V)-containing channels. When Xenopus oocytes expressing the MEC-4(d) channel complex are bathed in a CaCl2 solution, the Ca2+ current conducted by the MEC channel activates an oocyte-endogenous Ca2+-activated Cl− channel.13 The activation of the endogenous Ca2+-activated Cl− channel thus serves as an indirect measure of MEC channel Ca2+ permeability.
When we perfused oocytes expressing homomeric MEC-4(+) or homomeric MEC-4(A149V) channels with a CaCl2 solution, we did not observe the induction of the Ca2+-activated Cl− current, indicating that neither MEC-4(+) nor MEC-4(A149V) channels are permeable to significant Ca2+ currents in the absence of MEC-10(d) (Figure 6b). By contrast, we find that the MEC-4(A149V)+MEC-10(d) channel activates the Xenopus oocyte endogenous Ca2+-activated Cl− current significantly (Figure 6a and b),13 establishing that channels formed by MEC-4(A149V)+MEC-10(d) are Ca2+ permeable and, in this assay, showing that the neurotoxic channel conducts more Ca2+ than the WT channel. The level of current induction in the MEC-4(A149V)+MEC-10(d) channel is less than that induced by the MEC-4(d)+MEC-10(d) (measuring ∼20% of the MEC-4(d)+MEC-10(d) channel), paralleling in vivo data on relative toxicity of MEC-4(A149V)+MEC-10(d) versus MEC-4(d).
MEC-4(A149V)+MEC-10(d) channels exhibit enhanced Ca2+ currents without a change in Ca2+ permeability. (a) The same oocyte shown in Figure 5a was exposed to the Ca2+ solution, resulting in the activation of the endogenous Ca2+-activated Cl− current. (b) Average Ca2+-activated Cl− current at -160 mV for the subunit combinations indicated (+MEC-2 and MEC-6). Note that for MEC-4(A149V)+MEC-10(d), both Na+ currents and Ca2+-activated Cl− currents, which are a measure of the amount of Ca2+ permeating through the channel, are bigger than all the other channel combinations except MEC-4(d)+MEC-10(d). Data are expressed as mean±SE. **P<0.01 by comparison with all the other channel compositions, by t-test. (c) Average Na+ current at −160 mV from oocytes injected with MEC-4(d)+MEC-10(d) and MEC-4(A149V)+MEC-10(d) (+ MEC-2 and MEC-6). Note that in this batch of oocytes, Na+ currents produced by the expression of MEC-4(A149V)+MEC-10(d) are only 8% of currents produced by the expression of MEC-4(d)+MEC-10(d). (d) The average Ca2+ currents at −160 mV for the same subunit combinations shown in panel a. MEC-4(A149V)+MEC-10(d) channels produce Ca2+ currents that are 25% of currents produced by the expression of MEC-4(d)+MEC-10(d). We compared Na+ and Ca2+ current ratios by Student's t-test and we also found that the ratios to be statistically different (P⩽0.05). The difference in the size of the Na+ and Ca2+ currents as compared with MEC-4(d)+MEC-10(d) might suggest that MEC-4(A149V) affects permeability, conductance, or open probability of the channel complex when conducting Ca2+ and Na+ currents in two distinct ways. (e) Current–voltage relationship of Ca2+ currents is measured using the EGTA injection protocol in oocytes expressing MEC-4(d)+MEC-10(d) (open circles) and MEC-4(A149V)+MEC-10(d) (+MEC-2 and MEC-6). The reversal potentials of the Ca2+ currents were not statistically different (−65±5.07, n=5 versus −70±7.83, n=13, respectively). This result indicates that Ca2+ permeability is not affected by the A149V mutation, and thus either conductance or open probability may explain the elevated Ca2+ conductance
To examine Ca2+ currents of MEC-4(A149V)-containing channels more quantitatively, we perfused oocytes containing MEC-10(d) + either MEC-4(A149V) or MEC-4(d) with a CaCl2 solution and simultaneously blocked the endogenous Ca2+-activated Cl− current by injecting EGTA into the oocytes. Previously, we showed that the MEC-4(d) channels, but not the MEC-4(+) channels, induce a detectable Ca2+ current in this assay.13 When we measure the MEC channel Ca2+ current directly, we find that MEC-4(A149V)+MEC-10(d) Ca2+ currents are indeed detectable and are ∼25% of those of the MEC-4(d)+MEC-10(d) channel (Figure 6d). We conclude that the combinatorial MEC-4(A149V)+MEC-10(d) channel conducts elevated Ca2+, whereas channels including either MEC-4(+) or MEC-10(+) do not.
The MEC-4(A149V) substitution may impact channel permeability to Ca2+, open probability, or Ca2+ conductance. As a first step toward distinguishing among these possibilities, we determined the reversal potential of the current when oocytes were perfused with CaCl2. The reversal potential is dictated by the permeability of the permeating ions bathing the extracellular and intracellular side of the membrane (Na+ and Ca2+) and by their concentrations. We found that the reversal potential of currents produced by the expression of MEC-4(d)+MEC-10(d) channel was not statistically different from the reversal potential of currents produced by the expression of MEC-4(A149V)+MEC-10(d) channel (Figure 6e), indicating that Ca2+ permeability of the MEC-4(A149V)- and MEC-4(d)-containing channel complexes is similar. Remaining mechanistic possibilities are that channel conductance and/or open probability of MEC-4(A149V) channels may be different depending on the permeating ion, alternatives that can be addressed in single channel experiments.
Overall, our electrophysiological studies indicate that combinatorial neurotoxicity is correlated with elevated Ca2+ and Na+ currents in the MEC-4(A149V)+MEC-10(d) channel, by a mechanism probably associated with increased channel conductance and/or open probability.
Discussion
Here, we report on a DEG/ENaC channel hyperactivated by a novel mechanism in which two distinct channel subunit variants, each of which only marginally impacts channel activity on its own, interact to alter channel properties to a toxic end. Our data hold implications for in vivo structure relationships within the channel class and convey insight into the mechanisms of neurotoxicity likely to apply across phyla.
The MEC-4(A149V) subunit is neurotoxic only in combination with MEC-10(d)
Our genetic data establish that neither the homomeric MEC-4(A149V) channel nor the heteromeric MEC-4(A149V)+MEC-10(+) channel induces neurodegeneration in vivo, indicating that these channel configurations do not hyperactivate currents to neurotoxic levels. Remarkably, MEC-4(A149V) actually forms a functional touch-transducing complex in vivo as evidenced by the normal touch sensitivity of mec-4(bz301) mutants. We conclude that the basically functional MEC-4(A149V) subunit interacts with a borderline-deleterious MEC-10(d) subunit to induce significant neurotoxicity. This is the first case of synthetic neurotoxicity, dependent on two mutant subunits, reported for the DEG/ENaC channel class and a clear example in which interactions between subunits can dramatically alter channel activity. Given the demonstrated contributions of ASIC1a channels to infarct size in mouse ischemia models, and ENaC channels to human hypertension, our data raise the possibility that allelic interactions among DEG/ENaC subunits may differentially predispose individuals to neuronal loss in stroke or to blood pressure dysregulation, suggesting the potential importance of evaluating channel variants in patients affected by these conditions.
An extracellular AA substitution impacts channel pore and gating properties
Precise execution of ion channel conduction, critical to nervous system function and dysfunction, is intimately tied to channel structure, and our findings hold implications for structure/activity of the DEG/ENaC channel class in physiological context. MEC-4(A149) is an extracellular residue positioned 19 AAs away from the first transmembrane domain and adjacent to a conserved extracellular domain (MEC-4 AA 151–163; FPAITLCNLNPYK) (Figure 3a and b). Residues corresponding to MEC-4(A149) in other DEG/ENaC channel subunits are most commonly Ala or a hydrophobic residue (including Val, consistent with our finding that the MEC-4A149V mutant subunit is actually functional in vivo; see Figure 3b and Supplementary Figure 1). Little site-directed mutagenesis has probed the function of the adjacent FPAITLCNLNPYK region in the channel class, and no mec-4-disrupting mutations reported to date affect this region. Regions more C-terminal to the FPAITLCNLNPYK domain in mammalian DEG/ENaCs include a protease recognition site and a toxin-binding site (ASIC1a).21, 22, 23 Studies on these domains, as well as genetic analysis of a more distant domain in C. elegans MEC-4 and DEG-1 that negatively regulates channel activity,16 implicate multiple regions of the large extracellular domain of DEG/ENaCs in influencing channel properties.
How might extracellularly positioned MEC-4(A149) interact with the pore-associated MEC-10(d) subunit to alter channel conductance properties? The ASIC1a channel structure suggests that MEC-4(A149) and MEC-10(d) should not be in close proximity, at least in the desensitized state.3 Thus, AA changes at these sites might induce conformational changes transmitted across the channel structure. Indeed, MEC-4(A149) corresponds to ASIC1a(T87), which contributes to a β-sheet structure that forms intrasubunit and intersubunit solvent-filled cavities,3 and has been suggested to undergo extensive structural change during gating.24
Considering relative roles of Na+ and Ca2+ in neurotoxicity
In Xenopus oocytes, we found that the homomeric MEC-4(A149V) channel is permeable to Na+ but not to Ca2+. Na+ conductance in this mutant channel is approximately 10-fold elevated compared with the native MEC-4(+) channel (Figure 6b and Goodman et al.11), although the overall current is still small (∼1 μA). If there is a modest increase in Na+ current in the in vivo MEC-4(A149V) channel, it does not suffice to disrupt normal touch receptor function in the mec-4(bz301) mutant. Although we cannot rule out that channel properties in vivo might differ from those of the heterologously expressed channels, our observations suggest that (1) that MEC channels might not need to be permeable to Ca2+ ions to transduce touch sensation; and (2) larger currents through the MEC channels might not necessarily cause neurodegeneration if the channel is not permeable to Ca2+. Our data also suggest that AAs corresponding to the MEC-4(A149) position in ASIC and ENaC channels may contribute to modulating channel conductance properties.
Genetic and physiological studies have previously suggested that Ca2+ permeability might be a critical factor in MEC-4(d) channel neurotoxicity.13, 15 Although the combination channel is hyperactivated for both Na+ and Ca2+ currents, Ca2+ conductance appears increased disproportionately to Na+ conductance in the heteromeric MEC-4(A149V)+MEC-10(d) channel, as compared with the MEC-4(d)+MEC-10(d) channel (Na+ and Ca2+ currents are 8 and 25%, respectively, of currents generated by the expression of mec-4(d)+mec-10(d); Figure 6c and d). The relative change in ion conductance could result from an increase in Ca2+ conductance in the MEC-4(A149V) channel or an increase in Na+ conductance in the MEC-4(d) channels.
Although we cannot separate Na+ from Ca2+ conduction in these channels in vivo, our combined data suggest that Ca2+ permeability may be an essential factor in neurotoxicity. First, only the channels that exhibit significant Ca2+ currents in the oocyte expression system (MEC-4(d) and MEC-4(A149V)-containing channels) induce neurodegeneration in vivo,13 and this general observation extends to the neurotoxic ASIC1a channel.2, 14 Second, we note that the Na+ currents conducted by the MEC-4(A149V)+MEC-10(d) mutant channel are as large as Na+ currents produced by the MEC-4(A713V,G717E) mutant channel we previously characterized, which is not neurotoxic in vivo;13 the Ca2+ currents of the MEC-4(A149V)+MEC-10(d) mutant channel, however, are larger than that of MEC-4(A713V,G717E). Thus, our data may have identified a threshold calcium conductance that correlates with in vivo neurotoxicity.
Finally, we have shown that necrosis progression induced by the combined C. elegans mutant subunits requires calreticulin function for neurotoxicity. This requirement supports a necrotic rather than apoptotic death mechanism25 and suggests that the in vivo calcium conductance of the MEC-4(A149V)+MEC-10(d) channel is above the threshold for calcium-dependent catastrophic ER calcium release.
In summary, our data provide in vivo evidence in support of conformational changes exerted across the extracellular domain of DEG/ENaCs to impact multiple channel properties, contributing to the emerging picture of a dynamic and complex extracellular domain for the DEG/ENaC channel class. Our data also further support a role for elevated calcium conductance for the neurotoxic properties of dysregulated DEG/ENaC channels, and add mechanistic detail to an understanding of necrosis-initiating conditions, relevant to problems of neuronal loss in stroke and ischemia. Continued analysis of structure/activity in physiological context should expand the understanding of the potential for modulation of DEG/ENaC extracellular domains for potential therapeutic application.
Materials and Methods
Genetic strains and nematode growth
Nematode strains were maintained at 20°C on standard nematode growth medium (NGM) seeded with Escherichia coli strain OP50 as food source,26 unless otherwise stated. mec-4(d)=mec-4(u231) encodes the neurotoxic MEC-4(A713V) substitution8 and mec-10(d) encodes MEC-10(A673V).9 Strains used were WT Bristol N2; mec-4(u231) X (mec-4(d)) (19); mec-10(tm1552) X (mec-10-null); crt-1(bz29) (calreticulin null);15 ZB154 zdIs5[pmec-4GFP] I (25); TU2562 dpy-20(e1282) IV; uIs22[mec-3::gfp dpy-20(+)] (42); ZB2356 bzIs67[pmec-10mec-10(d)::GFP + pRF4(rol-6(su1006))] X, abbreviated in following list as bzIs67[mec-10(d)]; ZB2394 zdIs5; bzIs67[mec-10(d)]; ZB2374 bzIs75[pmec-4mec-10(d)::GFP + unc-119(+)] IV, abbreviated in following list as bzIs75[mec-10(d)]; ZB2451 zdIs5; uIs22 bzIs75[mec-10(d)]; ZB2513 bzEx170[pmec-4mec-4(bz301)+pRF4(rol-6(su1006))]; zdIs5; uIs22 bzIs75[mec-10(d)]; ZB2528 bzEx177[pmec-4mec-4(+) + pRF4(rol-6(su1006))]; zdIs5; uIs22 bzIs75[mec-10(d)]. Double-mutant strains were constructed by standard genetic approaches.
mec-10(tm1552) was provided by the Japan National Bioresources Consortium (Dr. Shohei Mitani, unpublished observations) and has a ∼0.45 kb deletion that removes sequences from exon 5 and part of exon 6, creating frameshift with a stop codon very close to the deletion site. No mec-10 transcripts can be detected by reverse transcription-polymerase chain reaction (RT-PCR) in this background. We used primers with sequence 5′-GTAGGGTCTGCAACTAGCTC-3′ and 5′-TGGGAGGGAGCTTCATCTTA-3′ to identify the deleted genome. This strain was outcrossed 6 × before analysis and further genetic constructions.
Strain ZB2356 bzIs67[pmec-10mec-10(d)::GFP + pRF4(rol-6(su1006))] X was constructed by co-injecting plasmid pmec-10mec-10(d)::GFP and pRF4(rol-6(su1006)) into the WT N2 strain, selecting roller transformants and X-ray irradiating transgenics to identify stably transformed lines as described.27 Integrated lines were outcrossed at least 6 × before further constructions or analysis. bzIs67 appeared X-linked because crossing bzIs67 males to N2 hermaphrodites yielded 50 : 50 males that were non-Rol. Single nucleotide polymorphism (SNIP) mapping28 positioned bzIs67 at approximately +10 on the X chromosome. The mutagenesis strain ZB2394 zdIs5[pmec-4GFP] I; bzIs67[pmec-10mec-10(d)::GFP + pRF4(rol-6(su1006))] X was constructed using standard genetic approaches. The strain ZB2374 bzIs75[pmec--4mec-10(d)::GFP+unc-119(+)] IV was created by the microparticle bombardment method as described.29 Integrated lines were outcrossed at least four times before further constructions. SNIP mapping28 positioned bzIs75 on chromosome IV. For the verification of the molecular identity of bz301, we injected pmec-4(bz301)+pRF4(rol-6(su1006)) and pmec-4(+)+pRF4(rol-6(su1006)) into ZB2451 zdIs5; uIs22 bzIs75[mec-10(d)] strain to make ZB2513 bzEx170[pmec-4mec-4(bz301)+pRF4(rol-6(su1006))]; zdIs5; uIs22 bzIs75[mec-10(d)] and ZB2528 bzEx177[pmec-4mec-4(+)+pRF4(rol-6(su1006))];zdIs5; uIs22 bzIs75[mec-10(d)].
Genetic screen for enhancers of mec-10(d)-induced cell death
We used nematode strain ZB2394, harboring the mec-10(d) transgene and expressing a GFP transgene (pmec-4GFP) exclusively in touch neurons in our screen for necrosis enhancers. In this strain, almost all six touch neurons survive and fluoresce. We mutagenized L4/young adult animals using EMS according to standard protocols.26 We distributed 30 F1 progeny to 15 cm × 15 cm plates and allowed animals to self-fertilize. Four days later, we screened F2 animals from each plate for the loss of touch cell fluorescence using a COPAS BIOSORT (Complex Object Parametric Analyzer and Sorter) from Union Biometrica (Holliston, MA, USA). We cloned out individuals with fewer than six fluorescent touch cells to create stocks of candidate homozygous suppressor mutants in which most of the population harbored <6 fluorescent touch cells (maximum of one line finally selected per mutagenesis plate to insure independent origin). We mapped bz301 using standard procedures26 (using bzIs67 as X chromosome marker) and we identified the nucleotide change associated by DNA sequence of PCR products (GENEWIZ Inc., South Plainfield, NJ, USA).
General microscopy and touch assay
We scored for PLM GFP signals by observing the tails of L4 stage larvae with fluorescence dissection microscopy. We scored for swollen necrotic-like PLM touch neurons by examining tails of L1 stage larvae with DIC microscopy as previously described.30 We took digital photographs through a Zeiss Axioplan 2 microscope after immobilizing L4 worms with 10 mM levamisol and placing on a 2% agarose pad. We performed gentle touch tests by stroking the body at anterior and posterior positions with an eyelash as described.7
Molecular biology
We constructed the pmec-10mec-10∷GFP plasmid by introducing a PstI–BamHI fragment, including the mec-10 promoter and coding sequences except for those encoding the last three AAs, into pPD95.77 vector, which includes enhanced GFP (Fire Lab Vector Kit31). We then used the Quick Change Site Directed Mutagenesis Kit from Stratagene to change the AA at position 673 to generate pmec-10mec-10(d)∷GFP (oligos used: 5′-GTAAAAATGATGGTTGATTTTGGAGGACACCTTGGACTTTGGTC-3′ and 5′-GACCAAAGTCCAAGGTGTCCTCCAAAATCAACCATCATTTTTAC-3′). We constructed pmec-4mec-10(d)∷GFP by subcloning a 1.02 kb StuI–ClaI fragment containing the mec-4 promoter into pmec-10mec-10(d)∷GFP to replace the mec-10 promoter. StuI–ClaI restriction sites on the fragment were introduced by PCR using the following primers: 5′-GAAGGCCTAAGCTTCAATACAAGCTCAAATAC-3′ and 5′-CCATCGATTCCCTCTATAACTTGATAGCGATA-3′. pmec-4 was described by Hong and Driscoll32 and constructed by subcloning a 6.1 kb HindIII–HindIII fragment containing genomic mec-4 promoter and coding sequences into pBluescript KS(−).8 We constructed the pmec-4(bz301) by site-directed mutagenesis (Quick Change Site Directed Mutagenesis Kit from Stratagene) using the pmec-4 plasmid as template (oligos used: 5′-CCAGACACTGTACCTTTTCCAGCAATTACGCTTTG-3′ and 5′-CAAAGCGTAATTGCTGGAAAAGGTACAGTGTCTGG-3′).
For channel assays in Xenopus oocytes, we used mec-2, mec-6, mec-4(d), and mec-10(d) cDNAs subcloned into pGEM-HE or pSGEM, a gift from the Chalfie Lab described by Chelur et al.,10 Goodman et al.,11 and Bianchi et al.13 We amplified cRNAs using the SMC4 bacterial strain.11 We introduced the MEC-4(A149V) substitution into the PGEM+mec-4 cDNA clone11 by site-directed mutagenesis (Quick Change Site Directed Mutagenesis Kit, Stratagene), using oligos: 5′-GAAATTTGACACTGTACCTTTTCCAGCAATTACGC-3′ and 5′-GCGTAATTGCTGGAAAAGGTACAGTGTCAAATTTC-3′.
Oocyte expression and electrophysiology
We synthesized capped RNAs using T7 mMESSAGE mMACHINE kit (Ambion), purified on Qiagen RNAeasy columns, and ran on denaturating agarose gels to check for size and cRNA integrity. We quantified cRNA by spectroscopy. We manually defolliculated stage V–VI oocytes after selecting them among multistaged oocytes dissected by 2 h collagenase treatment (2 mg/ml in Ca2+-free OR2 solution) from Xenopus laevis ovaries (NASCO). We incubated oocytes in OR2 media, which consists of 82.5 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM Na2HPO4, 0.5 g/l polyvinyl pyrolidone and 5 mM HEPES (pH 7.2), supplemented with penicillin and streptomycin (0.1 mg/ml) and 2 mM Na-pyruvate. The following day we injected oocytes with 52 nl of cRNA mix for a final amount of 5 ng per oocyte of each cRNA except for MEC-6, which we injected at the concentration of 1 ng per oocyte. We then incubated oocytes in OR2 at 20°C for 4 days before recording. We measured currents 4–10 days after cRNA injection using a two-electrode voltage clamp amplifier (GeneClamp 500B, Axon Instruments) at room temperature. Electrodes (0.3–1 M) were filled with 3 M KCl and we perfused oocytes with a NaCl solution containing (in mM): NaCl (100), KCl (2), CaCl2 (1), MgCl2 (2), HEPES (10), pH 7.2 or with a CaCl2 solution containing CaCl2 (73), KCl (2), HEPES (10), pH 7.2. We obtained chemicals from Sigma and Calbiochem. We used the pCLAMP suite of programs (Axon Instruments) for data acquisition and analysis. Currents were filtered at 200 Hz and sampled at 1 kHz.
Abbreviations
- AA:
-
amino acid
- ASICs:
-
acid-sensing ion channels
- COPAS BIOSORT:
-
Complex Object Parametric Analyzer and Sorter
- CRD:
-
Cys-rich domain
- DEG/ENaCs:
-
Degenerin/epithelial amiloride-sensitive Na+ channels
- EMS:
-
ethyl methyl sulfonate
- GFP:
-
green fluorescent protein
- L1–L4:
-
larval stages 1–4
- MEC:
-
mechanosensory
- MSD:
-
membrane-spanning domain
- N2:
-
wild-type C. elegans strain-Bristol isolate
- PLM:
-
posterior lateral microtubule neurons
- SNIP:
-
single nucleotide polymorphism
- WT:
-
wild type
References
Sattler R, Tymianski M . Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol 2001; 24: 107–129.
Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 2004; 118: 687–698.
Jasti J, Furukawa H, Gonzales EB, Gouaux E . Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 2007; 449: 316–323.
Kellenberger S, Schild L . Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 2002; 82: 735–767.
Suzuki H, Kerr R, Bianchi L, Frøkjaer-Jensen C, Slone D, Xue J et al. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron 2003; 39: 1005–1017.
O’Hagan R, Chalfie M, Goodman MB . The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci 2005; 8: 43–50.
Chalfie M, Sulston J . Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev Biol 1981; 82: 358–370.
Driscoll M, Chalfie M . The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature 1991; 349: 588–593.
Huang M, Chalfie M . Gene interactions affecting mechanosensory transduction in Caenorhabditis elegans. Nature 1994; 367: 467–470.
Chelur DS, Ernstrom GG, Goodman MB, Yao CA, Chen L, O’Hagan R et al. The mechanosensory protein MEC-6 is a subunit of the C. elegans touch-cell degenerin channel. Nature 2002; 420: 669–673.
Goodman MB, Ernstrom GG, Chelur DS, O’Hagan R, Yao CA, Chalfie M . MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature 2002; 415: 1039–1042.
Emtage L, Gu G, Hartwieg E, Chalfie M . Extracellular proteins organize the mechanosensory channel complex in C. elegans touch receptor neurons. Neuron 2004; 44: 795–807.
Bianchi L, Gerstbrein B, Frøkjaer-Jensen C, Royal DC, Mukherjee G, Royal MA et al. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nat Neurosci 2004; 7: 1337–1344.
Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ . Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci USA 2004; 101: 6752–6757.
Xu K, Tavernarakis N, Driscoll M . Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca(2+) release from the endoplasmic reticulum. Neuron 2001; 31: 957–971.
Garcia-Anoveros J, Ma C, Chalfie M . Regulation of Caenorhabditis elegans degenerin proteins by a putative extracellular domain. Curr Biol 1995; 5: 441–448.
Hong K, Mano I, Driscoll M . In vivo structure–function analyses of Caenorhabditis elegans MEC-4, a candidate mechanosensory ion channel subunit. J Neurosci 2000; 20: 2575–2588.
Royal DC, Bianchi L, Royal MA, Lizzio Jr M, Mukherjee G, Nunez YO et al. Temperature-sensitive mutant of the Caenorhabditis elegans neurotoxic MEC-4(d) DEG/ENaC channel identifies a site required for trafficking or surface maintenance. J Biol Chem 2005; 280: 41976–41986.
Bianchi L, Driscoll M . The molecular basis of touch sensation as modeled in Caenorhabditis elegans. In: Stephan Frings, Jonathan Bradley (eds) Transduction Channels in Sensory Cells, Wiley: Weinheim, Germany, 2004, pp 1–30.
Brown AL, Fernandez-Illescas SM, Liao Z, Goodman MB . Gain-of-function mutations in the MEC-4 DEG/ENaC sensory mechanotransduction channel alter gating and drug blockade. J Gen Physiol 2007; 129: 161–173.
Poirot O, Vukicevic M, Boesch A, Kellenberger S . Selective regulation of acid-sensing ion channel 1 by serine proteases. J Biol Chem 2004; 279: 38448–38457.
Vukicevic M, Weder G, Boillat A, Boesch A, Kellenberger S . Trypsin cleaves acid-sensing ion channel 1a in a domain that is critical for channel gating. J Biol Chem 2006; 281: 714–722.
Chen X, Kalbacher H, Grunder S . Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent. J Gen Physiol 2006; 127: 267–276.
Cushman KA, Marsh-Haffner J, Adelman JP, McCleskey EW . A conformation change in the extracellular domain that accompanies desensitization of acid-sensing ion channel (ASIC) 3. J Gen Physiol 2007; 129: 345–350.
Chung S, Gumienny TL, Hengartner MO, Driscoll M . A common set of engulfment genes mediates removal of both apoptotic and necrotic cell corpses in C. elegans. Nat Cell Biol 2000; 2: 931–937.
Brenner S . The genetics of Caenorhabditis elegans. Genetics 1974; 77: 71–94.
Rosenbluth RE, Cuddeford C, Baillie DL . Mutagenesis in Caenorhabditis elegans II. A spectrum of mutational events induced with 1500 r of gamma-radiation. Genetics 1985; 109: 493–511.
Wicks SR, Yeh RT, Gish WR, Waterston RH, Plasterk RH . Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat Genet 2001; 28: 160–164.
Praitis V, Casey E, Collar D, Austin J . Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 2001; 157: 1217–1226.
Driscoll M . Methods for the study of cell death in the nematode Caenorhabditis elegans. Methods Cell Biol 1995; 46: 323–353.
Mello C, Fire A . DNA transformation. Methods Cell Biol 1995; 48: 451–482.
Hong K, Driscoll M . A transmembrane domain of the putative channel subunit MEC-4 influences mechanotransduction and neurodegeneration in C.elegans. Nature 1994; 367: 470–473.
Acknowledgements
We thank the Japan National Bioresource Project and Dr. Shoehi Mitani for the mec-10 deletion, and Dr. Scott Clark for the zdIs5 line. The C. elegans Genetic Stock Center provided some strains used for mapping. This work was supported by research grants from the NJ Commission on Spinal Cord Research to WZ (04-2902-SCR-E-0 and 06-2916-SCR-E-0); NIH Grant R01 NS034435 to MD and NIH Grant R21 NS049511 to LB.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by P Nicotera
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, W., Bianchi, L., Lee, WH. et al. Intersubunit interactions between mutant DEG/ENaCs induce synthetic neurotoxicity. Cell Death Differ 15, 1794–1803 (2008). https://doi.org/10.1038/cdd.2008.114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2008.114
Keywords
This article is cited by
-
Loss of the RhoGAP SRGP-1 promotes the clearance of dead and injured cells in Caenorhabditis elegans
Nature Cell Biology (2011)