Abstract
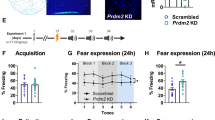
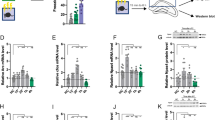
The mechanisms responsible for fear memory formation and extinction are far from being understood. Uncovering the molecules and mechanisms regulating these processes is vital for identifying molecular targets for the development of novel therapeutic strategies for anxiety and fear disorders. Cognitive abilities require the activation of gene expression necessary to the consolidation of lasting changes in neuronal function. In this study we established a key role for an epigenetic factor, the de novo DNA methyltransferase, Dnmt3a2, in memory formation and extinction. We found that Dnmt3a2 overexpression in the hippocampus of young adult mice induced memory enhancements in a variety of situations; it converted a weak learning experience into long-term memory, enhanced fear memory formation and facilitated fear memory extinction. Dnmt3a2 overexpression was also associated with the increased expression of plasticity-related genes. Furthermore, the knockdown of Dnmt3a2 expression impaired the animals’ ability to extinguish memories, identifying Dnmt3a2 as a key player in extinction. Thus, Dnmt3a2 is at the core of memory processes and represents a novel target for cognition-enhancing therapies to ameliorate anxiety and fear disorders and boost memory consolidation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ . Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther 2014; 149: 150–190.
Parsons RG, Ressler KJ . Implications of memory modulation for post-traumatic stress and fear disorders. Nat NeuroscI 2013; 16: 146–153.
Milad MR, Quirk GJ . Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 2012; 63: 129–151.
Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A . Neuronal circuits of fear extinction. Eur J Neurosci 2010; 31: 599–612.
Quirk GJ, Mueller D . Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 2008; 33: 56–72.
Myers KM, Davis M . Mechanisms of fear extinction. Mol Psychiatry 2007; 12: 120–150.
Orsini CA, Maren S . Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev 2012; 36: 1773–1802.
Kwapis JL, Wood MA . Epigenetic mechanisms in fear conditioning: implications for treating post-traumatic stress disorder. Trends Neurosci 2014; 37: 706–720.
Zovkic IB, Sweatt JD . Epigenetic mechanisms in learned fear: implications for PTSD. Neuropsychopharmacology 2013; 38: 77–93.
Portela A, Esteller M . Epigenetic modifications and human disease. Nat Biotechnol 2010; 28: 1057–1068.
Myers KM, Carlezon WA Jr, Davis M . Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology 2011; 36: 274–293.
Ganasen KA, Ipser JC, Stein DJ . Augmentation of cognitive behavioral therapy with pharmacotherapy. Psychiatr Clin North Am 2010; 33: 687–699.
Oliveira AM, Hemstedt TJ, Bading H . Rescue of aging-associated decline in Dnmt3a2 expression restores cognitive abilities. Nat Neurosci 2012; 15: 1111–1113.
Chen T, Ueda Y, Xie S, Li E . A novel Dnmt3a isoform produced from an alternative promoter localizes to euchromatin and its expression correlates with active de novo methylation. J Biol Chem 2002; 277: 38746–38754.
Zhang SJ, Steijaert MN, Lau D, Schutz G, Delucinge-Vivier C, Descombes P et al. Decoding NMDA receptor signaling: identification of genomic programs specifying neuronal survival and death. Neuron 2007; 53: 549–562.
Erdmann G, Schutz G, Berger S . Inducible gene inactivation in neurons of the adult mouse forebrain. BMC Neurosci 2007; 8: 63.
Corcoran KA, Desmond TJ, Frey KA, Maren S . Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci 2005; 25: 8978–8987.
Graff J, Joseph NF, Horn ME, Samiei A, Meng J, Seo J et al. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell 2014; 156: 261–276.
Rudenko A, Dawlaty MM, Seo J, Cheng AW, Meng J, Le T et al. Tet1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron 2013; 79: 1109–1122.
Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ . Induction of fear extinction with hippocampal-infralimbic BDNF. Science 2010; 328: 1288–1290.
Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, Quirk GJ . Hippocampal—prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology 2014; 39: 2161–2169.
Stafford JM, Raybuck JD, Ryabinin AE, Lattal KM . Increasing histone acetylation in the hippocampus-infralimbic network enhances fear extinction. Biol Psychiatry 2012; 72: 25–33.
Alberini CM, Kandel ER . The regulation of transcription in memory consolidation. Cold Spring Harbor perspect Biol 2015; 7: a021741.
Bading H . Nuclear calcium signalling in the regulation of brain function. Nat Rev 2013; 14: 593–608.
Fleischmann A, Hvalby O, Jensen V, Strekalova T, Zacher C, Layer LE et al. Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J Neurosci 2003; 23: 9116–9122.
Bramham CR, Worley PF, Moore MJ, Guzowski JF . The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci 2008; 28: 11760–11767.
Okuno H . Regulation and function of immediate-early genes in the brain: beyond neuronal activity markers. Neurosci Res 2011; 69: 175–186.
Suzuki MM, Bird A . DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet 2008; 9: 465–476.
Wu H, Coskun V, Tao J, Xie W, Ge W, Yoshikawa K et al. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science 2010; 329: 444–448.
Day JJ, Childs D, Guzman-Karlsson MC, Kibe M, Moulden J, Song E et al. DNA methylation regulates associative reward learning. Nat Neurosci 2013; 16: 1445–1452.
Jones PA . Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 2012; 13: 484–492.
Guo JU, Ma DK, Mo H, Ball MP, Jang MH, Bonaguidi MA et al. Neuronal activity modifies the DNA methylation landscape in the adult brain. Nat Neurosci 2011; 14: 1345–1351.
Ooi SK, Qiu C, Bernstein E, Li K, Jia D, Yang Z et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature 2007; 448: 714–717.
Otani J, Nankumo T, Arita K, Inamoto S, Ariyoshi M, Shirakawa M . Structural basis for recognition of H3K4 methylation status by the DNA methyltransferase 3A ATRX-DNMT3-DNMT3L domain. EMBO Rep 2009; 10: 1235–1241.
Zhang Y, Jurkowska R, Soeroes S, Rajavelu A, Dhayalan A, Bock I et al. Chromatin methylation activity of Dnmt3a and Dnmt3a/3L is guided by interaction of the ADD domain with the histone H3 tail. Nucleic Acids Res 2010; 38: 4246–4253.
Baubec T, Colombo DF, Wirbelauer C, Schmidt J, Burger L, Krebs AR et al. Genomic profiling of DNA methyltransferases reveals a role for DNMT3B in genic methylation. Nature 2015; 520: 243–247.
Guo X, Wang L, Li J, Ding Z, Xiao J, Yin X et al. Structural insight into autoinhibition and histone H3-induced activation of DNMT3A. Nature 2015; 517: 640–644.
Whittle N, Singewald N . HDAC inhibitors as cognitive enhancers in fear, anxiety and trauma therapy: where do we stand? Biochem Soc Trans 2014; 42: 569–581.
Lattal KM, Barrett RM, Wood MA . Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci 2007; 121: 1125–1131.
Bredy TW, Barad M . The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem 2008; 15: 39–45.
Acknowledgements
We thank Dr AM Hagenston for comments on the manuscript and Stephanie Rothe for her contribution during the initial stages of this project. We thank Kübra Gülmez and Benjamin Zeuch for technical help during the revision stage of the manuscript. This work was supported by the Sonderforschungsbereich (SFB) 636 of the Deutsche Forschungsgemeinschaft (DFG) and an ERC Advanced Grant. HB and AMMO are members of the Excellence Cluster CellNetworks at Heidelberg University. AMMO is a recipient of an Emmy Noether grant from the DFG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Oliveira, A., Hemstedt, T., Freitag, H. et al. Dnmt3a2: a hub for enhancing cognitive functions. Mol Psychiatry 21, 1130–1136 (2016). https://doi.org/10.1038/mp.2015.175
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.175
This article is cited by
-
Synaptic control of DNA methylation involves activity-dependent degradation of DNMT3A1 in the nucleus
Neuropsychopharmacology (2020)
-
Neuronal ensemble-specific DNA methylation strengthens engram stability
Nature Communications (2020)
-
DNA methyltransferase isoforms expression in the temporal lobe of epilepsy patients with a history of febrile seizures
Clinical Epigenetics (2019)
-
How the epigenome integrates information and reshapes the synapse
Nature Reviews Neuroscience (2019)
-
Absence of genomic hypomethylation or regulation of cytosine-modifying enzymes with aging in male and female mice
Epigenetics & Chromatin (2016)