Abstract
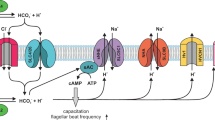
In mammals, sperm cells become motile during ejaculation and swim up the female reproductive tract. Before fertilization and to overcome various barriers, their motility must be hyperactivated, a motion that is characterized by vigorous asymmetric tail beating1. Hyperactivation requires an increase in calcium in the flagella, a process that probably involves plasmalemmal ion channels2,3,4,5,6,7,8. Numerous attempts in the past two decades to understand sperm cell channels have been frustrated by the difficulty of measuring spermatozoan transmembrane ion currents2,3,9,10,11,12,13,14,15,16. Here, by using a simple approach to patch-clamp spermatozoa and to characterize whole-spermatozoan currents, we describe a constitutively active flagellar calcium channel that is strongly potentiated by intracellular alkalinization. This current is not present in spermatozoa lacking the sperm-specific putative ion channel protein, CatSper1. This plasma membrane protein of the six transmembrane-spanning ion channel superfamily is specifically localized to the principal piece of the sperm tail and is required for sperm cell hyperactivation and male fertility4,5. Our results identify CatSper1 as a component of the key flagellar calcium channel, and suggest that intracellular alkalinization potentiates CatSper current to increase intraflagellar calcium and induce sperm hyperactivation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ho, H. C. & Suarez, S. S. Hyperactivation of mammalian spermatozoa: function and regulation. Reproduction 122, 519–526 (2001)
Darszon, A., Labarca, P., Nishigaki, T. & Espinosa, F. Ion channels in sperm physiology. Physiol. Rev. 79, 481–510 (1999)
Darszon, A. et al. Calcium channels and Ca2+ fluctuations in sperm physiology. Int. Rev. Cytol. 243, 79–172 (2005)
Ren, D. et al. A sperm ion channel required for sperm motility and male fertility. Nature 413, 603–609 (2001)
Carlson, A. E. et al. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl Acad. Sci. USA 100, 14864–14868 (2003)
Carlson, A. E. et al. Identical phenotypes of CatSper1 and CatSper2 null sperm. J. Biol. Chem. 280, 32238–32244 (2005)
Quill, T. A., Ren, D., Clapham, D. E. & Garbers, D. L. A voltage-gated ion channel expressed specifically in spermatozoa. Proc. Natl Acad. Sci. USA 98, 12527–12531 (2001)
Quill, T. A. et al. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc. Natl Acad. Sci. USA 100, 14869–14874 (2003)
Babcock, D. F., Bosma, M. M., Battaglia, D. E. & Darszon, A. Early persistent activation of sperm K+ channels by the egg peptide speract. Proc. Natl Acad. Sci. USA 89, 6001–6005 (1992)
Espinosa, F. et al. Mouse sperm patch-clamp recordings reveal single Cl- channels sensitive to niflumic acid, a blocker of the sperm acrosome reaction. FEBS Lett. 426, 47–51 (1998)
Gu, Y., Kirkman-Brown, J. C., Korchev, Y., Barratt, C. L. & Publicover, S. J. Multi-state, 4-aminopyridine-sensitive ion channels in human spermatozoa. Dev. Biol. 274, 308–317 (2004)
Guerrero, A., Sanchez, J. A. & Darszon, A. Single-channel activity in sea urchin sperm revealed by the patch-clamp technique. FEBS Lett. 220, 295–298 (1987)
Sanchez, D., Labarca, P. & Darszon, A. Sea urchin sperm cation-selective channels directly modulated by cAMP. FEBS Lett. 503, 111–115 (2001)
Gorelik, J. et al. Ion channels in small cells and subcellular structures can be studied with a smart patch-clamp system. Biophys. J. 83, 3296–3303 (2002)
Weyand, I. et al. Cloning and functional expression of a cyclic-nucleotide-gated channel from mammalian sperm. Nature 368, 859–863 (1994)
Florman, H. M., Arnoult, C., Kazam, I. G., Li, C. & O'Toole, C. M. A perspective on the control of mammalian fertilization by egg-activated ion channels in sperm: a tale of two channels. Biol. Reprod. 59, 12–16 (1998)
Lobley, A., Pierron, V., Reynolds, L., Allen, L. & Michalovich, D. Identification of human and mouse CatSper3 and CatSper4 genes: characterisation of a common interaction domain and evidence for expression in testis. Reprod. Biol. Endocrinol. 1, 53 (2003)
Cooper, T. G. Cytoplasmic droplets: the good, the bad or just confusing? Hum. Reprod. 20, 9–11 (2005)
Gonzalez-Martinez, M. T. Induction of a sodium-dependent depolarization by external calcium removal in human sperm. J. Biol. Chem. 278, 36304–36310 (2003)
Espinosa, F. & Darszon, A. Mouse sperm membrane potential: changes induced by Ca2+. FEBS Lett. 372, 119–125 (1995)
Horn, R. & Marty, A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol. 92, 145–159 (1988)
Hille, B. Ion Channels of Excitable Membranes (Sinauer Associates, Sunderland, MA, 2001)
Sather, W. A. & McCleskey, E. W. Permeation and selectivity in calcium channels. Annu. Rev. Physiol. 65, 133–159 (2003)
Fraire-Zamora, J. J. & Gonzalez-Martinez, M. T. Effect of intracellular pH on depolarization-evoked calcium influx in human sperm. Am. J. Physiol. Cell. Physiol. 287, C1688–C1696 (2004)
Santi, C. M., Santos, T., Hernandez-Cruz, A. & Darszon, A. Properties of a novel pH-dependent Ca2+ permeation pathway present in male germ cells with possible roles in spermatogenesis and mature sperm function. J. Gen. Physiol. 112, 33–53 (1998)
Babcock, D. F. Examination of the intracellular ionic environment and of ionophore action by null point measurements employing the fluorescein chromophore. J. Biol. Chem. 258, 6380–6389 (1983)
Zeng, Y., Oberdorf, J. A. & Florman, H. M. pH regulation in mouse sperm: identification of Na+-, Cl-, and HCO3--dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev. Biol. 173, 510–520 (1996)
Ho, H. C., Granish, K. A. & Suarez, S. S. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev. Biol. 250, 208–217 (2002)
Acknowledgements
We thank H. Qi for help in preparation of sperm cells and for discussion.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
D.E.C. has a patent pending on the use of CatSper1 as a contraception target.
Supplementary information
Supplementary Figure 1.
Monovalent ICatSper is potentiated by intracellular alkalinization. (PDF 272 kb)
Supplementary Figure 2.
CatSper conducts inward and outward Ba2+ current in response to a voltage ramp in symmetrical 50 mM [Ba2+]. (PDF 279 kb)
Supplementary Figure 3.
ICatSper is insensitive to cyclic nucleotides. (PDF 676 kb)
Supplementary Movie
Application of the patch pipette to the small cytoplasmic droplet of the sperm, a remnant of the precursor germ cell cytoplasm located on the midpiece of a mature sperm cell that is shed around the time of ejaculation. (AVI 7515 kb)
Supplementary Legends
Legends to accompany the above Supplementary Figures and Supplementary Movie. (DOC 26 kb)
Rights and permissions
About this article
Cite this article
Kirichok, Y., Navarro, B. & Clapham, D. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 439, 737–740 (2006). https://doi.org/10.1038/nature04417
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04417
This article is cited by
-
Vertebrate OTOP1 is also an alkali-activated channel
Nature Communications (2023)
-
Control of intracellular pH and bicarbonate by CO2 diffusion into human sperm
Nature Communications (2023)
-
An ion transporter in sperm that has features of a channel
Nature (2023)
-
Association of CATSPER1, SPATA16 and TEX11 genes polymorphism with idiopathic azoospermia and oligospermia risk in Iranian population
BMC Medical Genomics (2022)
-
Zinc is a master-regulator of sperm function associated with binding, motility, and metabolic modulation during porcine sperm capacitation
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.