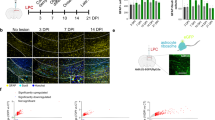
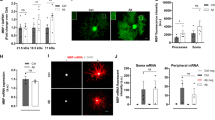
Abstract
Oligodendroglia support axon survival and function through mechanisms independent of myelination, and their dysfunction leads to axon degeneration in several diseases. The cause of this degeneration has not been determined, but lack of energy metabolites such as glucose or lactate has been proposed. Lactate is transported exclusively by monocarboxylate transporters, and changes to these transporters alter lactate production and use. Here we show that the most abundant lactate transporter in the central nervous system, monocarboxylate transporter 1 (MCT1, also known as SLC16A1), is highly enriched within oligodendroglia and that disruption of this transporter produces axon damage and neuron loss in animal and cell culture models. In addition, this same transporter is reduced in patients with, and in mouse models of, amyotrophic lateral sclerosis, suggesting a role for oligodendroglial MCT1 in pathogenesis. The role of oligodendroglia in axon function and neuron survival has been elusive; this study defines a new fundamental mechanism by which oligodendroglia support neurons and axons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Trapp, B. D. et al. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 338, 278–285 (1998)
Garbern, J. Y. et al. Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain 125, 551–561 (2002)
Griffiths, I. et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science 280, 1610–1613 (1998)
Lappe-Siefke, C. et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nature Genet. 33, 366–374 (2003)
Fünfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012)
Pierre, K. & Pellerin, L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J. Neurochem. 94, 1–14 (2005)
Koehler-Stec, E. M., Simpson, I. A., Vannucci, S. J., Landschulz, K. T. & Landschulz, W. H. Monocarboxylate transporter expression in mouse brain. Am. J. Physiol. 275, E516–E524 (1998)
Pierre, K., Pellerin, L., Debernardi, R., Riederer, B. M. & Magistretti, P. J. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience 100, 617–627 (2000)
Halestrap, A. P. & Price, N. T. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem. J. 343, 281–299 (1999)
Rinholm, J. E. et al. Regulation of oligodendrocyte development and myelination by glucose and lactate. J. Neurosci. 31, 538–548 (2011)
Pellerin, L. & Magistretti, P. J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl Acad. Sci. USA 91, 10625–10629 (1994)
Walz, W. & Mukerji, S. Lactate production and release in cultured astrocytes. Neurosci. Lett. 86, 296–300 (1988)
Pellerin, L. et al. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev. Neurosci. 20, 291–299 (1998)
Berthet, C. et al. Neuroprotective role of lactate after cerebral ischemia. J. Cereb. Blood Flow Metab. 29, 1780–1789 (2009)
Suzuki, A. et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823 (2011)
Benarroch, E. E. Oligodendrocytes: susceptibility to injury and involvement in neurologic disease. Neurology 72, 1779–1785 (2009)
Yamanaka, K. et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nature Neurosci. 11, 251–253 (2008)
Kang, S. H., Fukaya, M., Yang, J. K., Rothstein, J. D. & Bergles, D. E. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68, 668–681 (2010)
Pellerin, L., Pellegri, G., Martin, J. L. & Magistretti, P. J. Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc. Natl Acad. Sci. USA 95, 3990–3995 (1998)
Chiry, O. et al. Expression of the monocarboxylate transporter MCT1 in the adult human brain cortex. Brain Res. 1070, 65–70 (2006)
Gerhart, D. Z., Enerson, B. E., Zhdankina, O. Y., Leino, R. L. & Drewes, L. R. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am. J. Physiol. 273, E207–E213 (1997)
Regan, M. R. et al. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J. Neurosci. 27, 6607–6619 (2007)
Zhou, Q., Wang, S. & Anderson, D. J. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron 25, 331–343 (2000)
Hanu, R., McKenna, M., O’Neill, A., Resneck, W. G. & Bloch, R. J. Monocarboxylic acid transporters, MCT1 and MCT2, in cortical astrocytes in vitro and in vivo. Am. J. Physiol. Cell Physiol. 278, C921–C930 (2000)
Pellerin, L., Bergersen, L. H., Halestrap, A. P. & Pierre, K. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J. Neurosci. Res. 79, 55–64 (2005)
Bergersen, L., Rafiki, A. & Ottersen, O. P. Immunogold cytochemistry identifies specialized membrane domains for monocarboxylate transport in the central nervous system. Neurochem. Res. 27, 89–96 (2002)
Murray, C. M. et al. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nature Chem. Biol. 1, 371–376 (2005)
Suh, S. W. et al. Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmet hyl)propyl]-1H-indole-2-carboxamide). J. Pharmacol. Exp. Ther. 321, 45–50 (2007)
Heyer, E. J., Nowak, L. M. & Macdonald, R. L. Membrane depolarization and prolongation of calcium-dependent action potentials of mouse neurons in cell culture by two convulsants: bicuculline and penicillin. Brain Res. 232, 41–56 (1982)
Mayer, M. L. & Westbrook, G. L. Mixed-agonist action of excitatory amino acids on mouse spinal cord neurones under voltage clamp. J. Physiol. (Lond.) 354, 29–53 (1984)
Edgar, J. M. et al. Early ultrastructural defects of axons and axon-glia junctions in mice lacking expression of Cnp1. Glia 57, 1815–1824 (2009)
Morrison, B. M., Shu, I. W., Wilcox, A. L., Gordon, J. W. & Morrison, J. H. Early and selective pathology of light chain neurofilament in the spinal cord and sciatic nerve of G86R mutant superoxide dismutase transgenic mice. Exp. Neurol. 165, 207–220 (2000)
Sasaki, S. & Maruyama, S. Increase in diameter of the axonal initial segment is an early change in amyotrophic lateral sclerosis. J. Neurol. Sci. 110, 114–120 (1992)
Tekkök, S. B., Brown, A. M., Westenbroek, R., Pellerin, L. & Ransom, B. R. Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J. Neurosci. Res. 81, 644–652 (2005)
Ventura, A. et al. Cre-lox-regulated conditional RNA interference from transgenes. Proc. Natl Acad. Sci. USA 101, 10380–10385 (2004)
Doerflinger, N. H., Macklin, W. B. & Popko, B. Inducible site-specific recombination in myelinating cells. Genesis 35, 63–72 (2003)
Kirk, P. et al. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 19, 3896–3904 (2000)
Sánchez-Abarca, L. I., Tabernero, A. & Medina, J. M. Oligodendrocytes use lactate as a source of energy and as a precursor of lipids. Glia 36, 321–329 (2001)
Rinholm, J. E. et al. Regulation of oligodendrocyte development and myelination by glucose and lactate. J. Neurosci. 31, 538–548 (2011)
Seilhean, D. et al. Accumulation of TDP-43 and α-actin in an amyotrophic lateral sclerosis patient with the K17I ANG mutation. Acta Neuropathol. 118, 561–573 (2009)
Brenner, M., Kisseberth, W. C., Su, Y., Besnard, F. & Messing, A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J. Neurosci. 14, 1030–1037 (1994)
Yang, Y. et al. Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia 59, 200–207 (2011)
Doyle, J. P. et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell 135, 749–762 (2008)
Buntinx, M. et al. Characterization of three human oligodendroglial cell lines as a model to study oligodendrocyte injury: morphology and oligodendrocyte-specific gene expression. J. Neurocytol. 32, 25–38 (2003)
You, F., Osawa, Y., Hayashi, S. & Nakashima, S. Immediate early gene IEX-1 induces astrocytic differentiation of U87-MG human glioma cells. J. Cell. Biochem. 100, 256–265 (2007)
Maekawa, F., Minehira, K., Kadomatsu, K. & Pellerin, L. Basal and stimulated lactate fluxes in primary cultures of astrocytes are differentially controlled by distinct proteins. J. Neurochem. 107, 789–798 (2008)
Rothstein, J. D., Jin, L., Dykes-Hoberg, M. & Kuncl, R. W. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc. Natl Acad. Sci. USA 90, 6591–6595 (1993)
McIver, S. R. et al. Lentiviral transduction of murine oligodendrocytes in vivo. J. Neurosci. Res. 82, 397–403 (2005)
Yang, Y., Gozen, O., Vidensky, S., Robinson, M. B. & Rothstein, J. D. Epigenetic regulation of neuron-dependent induction of astroglial synaptic protein GLT1. Glia 58, 277–286 (2010)
Acknowledgements
We thank C. Coccia, S. Vidensky, I. Shats, L. Chakravarti, Y. Ayukawa and L. Mamedova for technical support, E. Potter for oligodendrocyte cultures, C. Cooke for electron microscopy, and S. Kang and D. Bergles for providing MOBP–eGFP BAC and CNP BacTrap mice, CASPR and Nav1.6 antibodies. Autopsy specimens were provided by the Johns Hopkins ALS Tissue Bank and the Johns Hopkins University Brain Resource Center supported by National Institutes of Health grants P50AG05146 and PO1NS16375. Support was provided by the Muscular Dystrophy Association (B.M.M. and Yo.L.), NIH-NS33958 (J.D.R.), P2ALS (J.D.R.), Packard Center for ALS (J.D.R.), Human Frontier Science Program-RG118/1998-B (L.P.), Swiss Fonds National de Recherche Scientifique-31003A-125063 (L.P.), Swiss National Science Foundation (FNRS)-3100AO-108336/1 (P.J.M.), Biaggi and Puccini Foundations (P.J.M). We dedicate this manuscript to J. W. Griffin, who passed away while this manuscript was under revision, and are grateful for his contributions to this manuscript and mentorship to many of the authors on this paper.
Author information
Authors and Affiliations
Contributions
All of the authors contributed to the design of the experiments. MCT1 BAC reporter experiments were designed and performed by Yo.L., L.J. and P.-W.Z. MCT1 ASO, MCT1i and human western blot experiments were designed and performed by B.M.M., Yu.L., A.T., Yi.L. and J.D.R. Lentiviral experiments were designed and performed by Yo.L., B.M.M., Yu.L. and J.D.R. The heterozygous MCT1-null mice were produced by S.L., L.P. and P.J.M., and analysed by Yo.L. Electron miscroscopy work was completed by M.H.F., Yo.L., B.M.M. and J.D.R. Optic nerve lentiviral injections were performed by P.N.H. The manuscript and figures were prepared by B.M.M. and J.D.R. with input from co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-13 and Supplementary Table 1. (PDF 22485 kb)
Rights and permissions
About this article
Cite this article
Lee, Y., Morrison, B., Li, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012). https://doi.org/10.1038/nature11314
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11314
This article is cited by
-
GBA1 inactivation in oligodendrocytes affects myelination and induces neurodegenerative hallmarks and lipid dyshomeostasis in mice
Molecular Neurodegeneration (2024)
-
Mechanosensitive channel of large conductance enhances the mechanical stretching-induced upregulation of glycolysis and oxidative metabolism in Schwann cells
Cell Communication and Signaling (2024)
-
Oligodendroglial metabolic support of myelinated axons driven by K+ dynamics
Nature Neuroscience (2024)
-
Oligodendrocyte–axon metabolic coupling is mediated by extracellular K+ and maintains axonal health
Nature Neuroscience (2024)
-
The role of TSC1 and TSC2 proteins in neuronal axons
Molecular Psychiatry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.