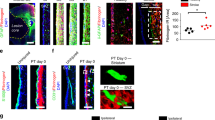
Abstract
Postnatal/adult neural stem cells (NSCs) within the rodent subventricular zone (SVZ; also called subependymal zone) generate doublecortin (Dcx)+ neuroblasts that migrate and integrate into olfactory bulb circuitry1,2. Continuous production of neuroblasts is controlled by the SVZ microenvironmental niche3,4. It is generally thought that enhancing the neurogenic activities of endogenous NSCs may provide needed therapeutic options for disease states and after brain injury. However, SVZ NSCs can also differentiate into astrocytes. It remains unclear whether there are conditions that favour astrogenesis over neurogenesis in the SVZ niche, and whether astrocytes produced there have different properties compared with astrocytes produced elsewhere in the brain5. Here we show in mice that SVZ-generated astrocytes express high levels of thrombospondin 4 (Thbs4)6,7, a secreted homopentameric glycoprotein, in contrast to cortical astrocytes, which express low levels of Thbs4. We found that localized photothrombotic/ischaemic cortical injury initiates a marked increase in Thbs4hi astrocyte production from the postnatal SVZ niche. Tamoxifen-inducible nestin-creERtm4 lineage tracing demonstrated that it is these SVZ-generated Thbs4hi astrocytes, and not Dcx+ neuroblasts, that home-in on the injured cortex. This robust post-injury astrogenic response required SVZ Notch activation modulated by Thbs4 via direct Notch1 receptor binding and endocytosis to activate downstream signals, including increased Nfia transcription factor expression important for glia production8. Consequently, Thbs4 homozygous knockout mice (Thbs4KO/KO) showed severe defects in cortical-injury-induced SVZ astrogenesis, instead producing cells expressing Dcx migrating from SVZ to the injury sites. These alterations in cellular responses resulted in abnormal glial scar formation after injury, and significantly increased microvascular haemorrhage into the brain parenchyma of Thbs4KO/KO mice. Taken together, these findings have important implications for post-injury applications of endogenous and transplanted NSCs in the therapeutic setting, as well as disease states where Thbs family members have important roles9,10.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
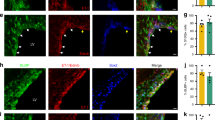
References
Kriegstein, A. & Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149–184 (2009)
Kelsch, W., Sim, S. & Lois, C. Watching synaptogenesis in the adult brain. Annu. Rev. Neurosci. 33, 131–149 (2010)
Ihrie, R. A. & Alvarez-Buylla, A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron 70, 674–686 (2011)
Paez-Gonzalez, P. et al. Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron 71, 61–75 (2011)
Molofsky, A. V. et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 26, 891–907 (2012)
Lawler, J. et al. Identification and characterization of thrombospondin-4, a new member of the thrombospondin gene family. J. Cell Biol. 120, 1059–1067 (1993)
Eroglu, C. The role of astrocyte-secreted matricellular proteins in central nervous system development and function. J. Cell Commun. Signal. 3, 167–176 (2009)
Deneen, B. et al. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron 52, 953–968 (2006)
Adams, J. C. & Lawler, J. The thrombospondins. Cold Spring Harb. Perspect. Biol. 3, a009712 (2011)
Liauw, J. et al. Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J. Cereb. Blood Flow Metab. 28, 1722–1732 (2008)
Kuo, C. T. et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell 127, 1253–1264 (2006)
Frolova, E. G. et al. Thrombospondin-4 regulates vascular inflammation and atherogenesis. Circ. Res. 107, 1313–1325 (2010)
Thored, P. et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 24, 739–747 (2006)
Givogri, M. I. et al. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev. Neurosci. 28, 81–91 (2006)
Li, L. et al. Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia 58, 1610–1619 (2010)
Maxwell, K. A. & Dyck, R. H. Induction of reproducible focal ischemic lesions in neonatal mice by photothrombosis. Dev. Neurosci. 27, 121–126 (2005)
Aguirre, A., Rubio, M. E. & Gallo, V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature 467, 323–327 (2010)
Morrison, S. J. et al. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101, 499–510 (2000)
Han, H. et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int. Immunol. 14, 637–645 (2002)
Carlén, M. et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nature Neurosci. 12, 259–267 (2009)
Meng, H., Zhang, X., Hankenson, K. D. & Wang, M. M. Thrombospondin 2 potentiates notch3/jagged1 signaling. J. Biol. Chem. 284, 7866–7874 (2009)
Yamamoto, S., Charng, W. L. & Bellen, H. J. Endocytosis and intracellular trafficking of Notch and its ligands. Curr. Top. Dev. Biol. 92, 165–200 (2010)
Namihira, M. et al. Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev. Cell 16, 245–255 (2009)
Moseley, M. E., de Crespigny, A. J., Roberts, T. P., Kozniewska, E. & Kucharczyk, J. Early detection of regional cerebral ischemia using high-speed MRI. Stroke 24, I60–I65 (1993)
Patel, M. R., Edelman, R. R. & Warach, S. Detection of hyperacute primary intraparenchymal hemorrhage by magnetic resonance imaging. Stroke 27, 2321–2324 (1996)
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010)
Faulkner, J. R. et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 24, 2143–2155 (2004)
Robel, S., Berninger, B. & Gotz, M. The stem cell potential of glia: lessons from reactive gliosis. Nature Rev. Neurosci. 12, 88–104 (2011)
Aboody, K., Capela, A., Niazi, N., Stern, J. H. & Temple, S. Translating stem cell studies to the clinic for CNS repair: current state of the art and the need for a Rosetta Stone. Neuron 70, 597–613 (2011)
Alcantara Llaguno, S. et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell 15, 45–56 (2009)
Scheffler, B. et al. Phenotypic and functional characterization of adult brain neuropoiesis. Proc. Natl Acad. Sci. USA 102, 9353–9358 (2005)
Christopherson, K. S. et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433 (2005)
Macia, E. et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10, 839–850 (2006)
Platel, J. C. et al. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron 65, 859–872 (2010)
McDowell, K. A. et al. Reduced cortical BDNF expression and aberrant memory in Carf knock-out mice. J. Neurosci. 30, 7453–7465 (2010)
Lee, J. K. et al. Photochemically induced cerebral ischemia in a mouse model. Surg. Neurol. 67, 620–625 (2007)
Johnson, G. A., Cofer, G. P., Gewalt, S. L. & Hedlund, L. W. Morphologic phenotyping with MR microscopy: the visible mouse. Radiology 222, 789–793 (2002)
Basser, P. J., Mattiello, J. & LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 66, 259–267 (1994)
Li, W., Wu, B. & Liu, C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. Neuroimage 55, 1645–1656 (2011)
Acknowledgements
We thank D. Melton (Harvard) for R26R-NICD mice; T. Honjo (Kyoto) for RBPjk-flox mice; F. Wang for R26R-tdTomato mice; E. Rawlins (Cambridge) for Foxj1-creERt2 mice; B. Deneen (B.C.M.) and S. Singh for discussions; G. Lyons, R. Andersen, P. Heine, D. Fromme and S. Collins for project assistance; Duke Flow Cytometry Facility for help with FACS; W. Li and Duke Center for In vivo microscopy/brain imaging for MRI analyses; and T. Lechler, A. West and B. Hogan for comments on manuscript. This work was supported by National Biomedical Technology Resource Center Grant P41 RR005959 (C.L.) of P41 EB015897 to Duke Center for In Vivo Microscopy; George and Jean Brumley Endowment, Sontag Foundation, David and Lucile Packard Foundation, March of Dimes, and NIH Director’s New Innovator Award 1 DP2 OD004453-01 (C.T.K.).
Author information
Authors and Affiliations
Contributions
E.J.B. performed injury and biochemical experiments; D.L. performed gene expression and live-imaging experiments; K.A. performed in vivo immunoprecipitation experiments; P.P.-G. performed SVZ antibody staining and analyses; R.J., H.S. and D.S.W. assisted with injuries and their analyses; C.L. performed MRI scanning and quantitative analyses; C.E. provided reagents and experimental insight; C.T.K. performed transplantations and conceived the project. E.J.B., D.L. and R.J. assembled figures and C.T.K. wrote the paper. All authors discussed results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-14. (PDF 7408 kb)
Live-imaging of lineage-traced rostral migratory stream Neuroblasts
This movie shows representative live-imaging of tdTomato+ rostral migratory stream (RMS) neuroblasts in acute brain slices, from N4; R26R-tdTomato mice induced with tamoxifen at P6 and imaged 3 weeks later. Left = rostral. Bidirectional travel of RMS neuroblasts in acute slice preparations had been reported previously34. Images were captured once every 5 minutes; total time = 55 minutes. (MOV 6955 kb)
Rights and permissions
About this article
Cite this article
Benner, E., Luciano, D., Jo, R. et al. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature 497, 369–373 (2013). https://doi.org/10.1038/nature12069
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12069
This article is cited by
-
Subventricular zone cytogenesis provides trophic support for neural repair in a mouse model of stroke
Nature Communications (2023)
-
Directional induction of neural stem cells, a new therapy for neurodegenerative diseases and ischemic stroke
Cell Death Discovery (2023)
-
Transcription and proteome changes involved in re-innervation muscle following nerve crush in rats
BMC Genomics (2022)
-
IRES-mediated Wnt2 translation in apoptotic neurons triggers astrocyte dedifferentiation
npj Regenerative Medicine (2022)
-
The role of neural stem cells in regulating glial scar formation and repair
Cell and Tissue Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.