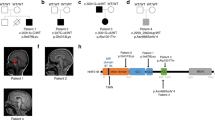
Abstract
Motor, sensory, and integrative activities of the brain are coordinated by a series of midline-bridging neuronal commissures whose development is tightly regulated. Here we report a new human syndrome in which these commissures are widely disrupted, thus causing clinical manifestations of horizontal gaze palsy, scoliosis, and intellectual disability. Affected individuals were found to possess biallelic loss-of-function mutations in the gene encoding the axon-guidance receptor 'deleted in colorectal carcinoma' (DCC), which has been implicated in congenital mirror movements when it is mutated in the heterozygous state but whose biallelic loss-of-function human phenotype has not been reported. Structural MRI and diffusion tractography demonstrated broad disorganization of white-matter tracts throughout the human central nervous system (CNS), including loss of all commissural tracts at multiple levels of the neuraxis. Combined with data from animal models, these findings show that DCC is a master regulator of midline crossing and development of white-matter projections throughout the human CNS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Edwards, T.J., Sherr, E.H., Barkovich, A.J. & Richards, L.J. Clinical, genetic and imaging findings identify new causes for corpus callosum development syndromes. Brain 137, 1579–1613 (2014).
Izzi, L. & Charron, F. Midline axon guidance and human genetic disorders. Clin. Genet. 80, 226–234 (2011).
Nugent, A.A., Kolpak, A.L. & Engle, E.C. Human disorders of axon guidance. Curr. Opin. Neurobiol. 22, 837–843 (2012).
Van Battum, E.Y., Brignani, S. & Pasterkamp, R.J. Axon guidance proteins in neurological disorders. Lancet Neurol. 14, 532–546 (2015).
Jen, J.C. et al. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science 304, 1509–1513 (2004).
Srour, M. et al. Mutations in DCC cause congenital mirror movements. Science 328, 592 (2010).
Depienne, C. et al. A novel DCC mutation and genetic heterogeneity in congenital mirror movements. Neurology 76, 260–264 (2011).
Méneret, A. et al. Congenital mirror movements: mutational analysis of RAD51 and DCC in 26 cases. Neurology 82, 1999–2002 (2014).
Kolodziej, P.A. et al. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell 87, 197–204 (1996).
Keino-Masu, K. et al. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87, 175–185 (1996).
Moore, S.W., Tessier-Lavigne, M. & Kennedy, T.E. Netrins and their receptors. Adv. Exp. Med. Biol. 621, 17–31 (2007).
Chen, Q. et al. N-terminal horseshoe conformation of DCC is functionally required for axon guidance and might be shared by other neural receptors. J. Cell Sci. 126, 186–195 (2013).
Griebel, M.L., Williams, J.P., Russell, S.S., Spence, G.T. & Glasier, C.M. Clinical and developmental findings in children with giant interhemispheric cysts and dysgenesis of the corpus callosum. Pediatr. Neurol. 13, 119–124 (1995).
Wahl, M., Barkovich, A.J. & Mukherjee, P. Diffusion imaging and tractography of congenital brain malformations. Pediatr. Radiol. 40, 59–67 (2010).
Sicotte, N.L. et al. Diffusion tensor MRI shows abnormal brainstem crossing fibers associated with ROBO3 mutations. Neurology 67, 519–521 (2006).
Kweldam, C.F. et al. Undecussated superior cerebellar peduncles and absence of the dorsal transverse pontine fibers: a new axonal guidance disorder? Cerebellum 13, 536–540 (2014).
Serafini, T. et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87, 1001–1014 (1996).
Harter, P.N. et al. Spatio-temporal deleted in colorectal cancer (DCC) and netrin-1 expression in human foetal brain development. Neuropathol. Appl. Neurobiol. 36, 623–635 (2010).
Fazeli, A. et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature 386, 796–804 (1997).
Fearon, E.R. et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science 247, 49–56 (1990).
Finger, J.H. et al. The netrin 1 receptors Unc5h3 and Dcc are necessary at multiple choice points for the guidance of corticospinal tract axons. J. Neurosci. 22, 10346–10356 (2002).
Fothergill, T. et al. Netrin-DCC signaling regulates corpus callosum formation through attraction of pioneering axons and by modulating Slit2-mediated repulsion. Cereb. Cortex 24, 1138–1151 (2014).
Srivatsa, S. et al. Unc5C and DCC act downstream of Ctip2 and Satb2 and contribute to corpus callosum formation. Nat. Commun. 5, 3708 (2014).
Lakhina, V. et al. Netrin/DCC signaling guides olfactory sensory axons to their correct location in the olfactory bulb. J. Neurosci. 32, 4440–4456 (2012).
Powell, A.W., Sassa, T., Wu, Y., Tessier-Lavigne, M. & Polleux, F. Topography of thalamic projections requires attractive and repulsive functions of Netrin-1 in the ventral telencephalon. PLoS Biol. 6, e116 (2008).
Braisted, J.E. et al. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. J. Neurosci. 20, 5792–5801 (2000).
Jarjour, A.A. et al. Maintenance of axo-oligodendroglial paranodal junctions requires DCC and netrin-1. J. Neurosci. 28, 11003–11014 (2008).
Rajasekharan, S. et al. Netrin 1 and Dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA. Development 136, 415–426 (2009).
Rabe, N., Gezelius, H., Vallstedt, A., Memic, F. & Kullander, K. Netrin-1-dependent spinal interneuron subtypes are required for the formation of left-right alternating locomotor circuitry. J. Neurosci. 29, 15642–15649 (2009).
Rabe Bernhardt, N. et al. DCC mediated axon guidance of spinal interneurons is essential for normal locomotor central pattern generator function. Dev. Biol. 366, 279–289 (2012).
Paul, L.K. et al. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat. Rev. Neurosci. 8, 287–299 (2007).
Jen, J. et al. Familial horizontal gaze palsy with progressive scoliosis maps to chromosome 11q23-25. Neurology 59, 432–435 (2002).
Sabatier, C. et al. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell 117, 157–169 (2004).
Zelina, P. et al. Signaling switch of the axon guidance receptor Robo3 during vertebrate evolution. Neuron 84, 1258–1272 (2014).
Yu, T.W. et al. Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat. Genet. 42, 1015–1020 (2010).
Thompson, C.L. et al. A high-resolution spatiotemporal atlas of gene expression of the developing mouse brain. Neuron 83, 309–323 (2014).
Lein, E.S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Acknowledgements
We thank A. Rozzo, J. Partlow, B. Barry, and R. Hill for logistical and administrative assistance; C. Carruthers, research assistant in FNNDSC, for creating and compressing the DTT movie files; and H. Somhegyi for expert illustrations. We thank the individuals and their families reported on herein for their participation in this research. This research was supported in part by the Repository Core for Neurological Disorders, Department of Neurology, Boston Children's Hospital, and the IDDRC (NIH P30HD018655). S.S.J. is supported by the National Medical Research Council, Singapore, and the Singhealth-Duke NUS Paediatric Academic Programme Nurturing Clinician Scientist Scheme. A.M.D. is supported by the National Institute of General Medical Sciences (T32GM07753) and a National Institutes of Health Ruth L. Kirschstein National Research Service Award (5T32 GM007226-39). E.C.E.'s contributions to this work were supported by NEI R01EY12498, IDDRC grant P30 HD018655, and the Manton Center for Orphan Disease Research. C.A.W. is supported by grants from the National Institute of Mental Health (R01MH083565), the National Institute of Neurological Disorders and Stroke (R01NS032457 and R01NS035129), the Simons Foundation, and the Manton Center for Orphan Disease Research. C.A.W. and E.C.E. are supported as Investigators of the Howard Hughes Medical Institute. T.W.Y.'s contributions to this work were supported by grants from the National Institute of Mental Health (R01MH083565), the Simons Foundation, and the Nancy Lurie Marks Foundation.
Author information
Authors and Affiliations
Contributions
S.S.J., E.C.E., C.A.W., and T.W.Y. designed experiments. K.S.-A. and K.M. performed the homozygosity and CNV analysis. S.S.J., A.M.D., A.-T.N.L., and S.S. performed the ddPCR, junction PCR, and RT–PCR. W.-M.C. performed Sanger sequencing of family 2. M.P., M.D., and P.E.G. performed the DTI and tractography analyses. M.R.D., N.J.C., M.G., Z.A.Z., M.A.D., A.A.J., K.A.-A., and T.M.B. performed the phenotypic assessment of the patients. A.J.B., C.A.W., M.D., M.P., C.D.R., I.A.A., P.E.G., E.C.E., and T.W.Y. reviewed the MRI imaging studies. W.W. performed the phenotypic assessment of patient 3, and E.S.L. performed the in situ hybridization analyses. S.S.J., E.C.E., C.A.W., and T.W.Y. wrote the manuscript. All coauthors reviewed and approved the final version of the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Additional clinicoradiographic features of DCC−/− syndrome.
(a) Horizontal gaze palsy as evidenced by failure of horizontal movements of the eye, with preservation of upward and downward gaze. (b-g) Agenesis of the corpus callosum. Axial and sagittal images showing ACC and variable interhemispheric cyst in Family 1: II:5 (b) and Family 1: II:7 (c). Axial view: control (d) vs. patient (Family 1: II:7) (f). Coronal view: control (e) vs. patient (Family 1: II:7) (g).
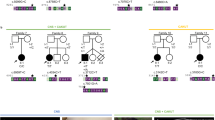
Supplementary Figure 2 Homozygosity mapping.
(a) Homozygosity analysis of the two affected individuals of Family 1 identified multiple regions of homozygosity across the genome. Observed homozygosity for the two siblings (II:5 and II:7) was 3.9% and 4.9% of the genome. (b) The observation is consistent with first and second cousin marriage (expectation 6.25% and 1.56%, respectively, as illustrated in the bottom figure; however, the distributions are broad, and there is not enough information to distinguish between the two). Note: only homozygous regions with 2 cM or longer are shown.
Supplementary Figure 3 Homozygosity mapping and copy-number analysis.
Analysis identified a block of homozygosity on chromosome 18, bounded by SNP markers rs10502546 and rs9950483 and included 139 UCSC genes (homozygous SNPs are shown in red or blue, heterozygous SNPs are shown in green, and SNPs for which no genotype could be assigned are shown in white). Copy number analysis (using Variant Explorer) identified a rare homozygous deletion (orange box) on chromosome 18q21.2 near exon 1 of DCC, and a common non-genic homozygous deletion on chromosome 18q12.3.
Supplementary Figure 4 Junction PCR.
(a) Digital droplet PCR using probes located within intron 1 of DCC (chr18: 49869550 (hg19) and chr18: 49871307 (hg19)) confirmed the homozygous deletion in both affected siblings (II:5 and II:7 from Family 1) and showed that their unaffected mother (I:2) was heterozygous for the deletion. (b) Schematic of the DCC gene with the predicted deletion near exon 1 (grey boxes). SNPs in blue indicate where copy number 2 was detected on the SNP array, and SNPs in green indicate where copy number 0 was detected on the SNP array, indicative of maximum and minimum boundaries of the predicted deletion, respectively. Nested PCR primers spanning the predicted deletion are indicated by F →, R ←. (c) Amplification with primers F5+R2 and F6+R2 yielded PCR products containing the deletion breakpoint in both the affected siblings (II:5 and II:7) and their mother (I:2), but not from an unrelated control individual. (d) Amplification with primers F2+R0 and F1+R0 yielded PCR products in the control sample, but non-specific bands in the affected individual II:7, confirming that the region can be amplified in the control sample.
Supplementary Figure 5 RT–PCR.
(a) Schematic representation of primers (forward →, reverse ←) used in DCC RT-PCR experiments to show selective loss of all products containing exon 1 in the affected individual II:7 (from Family 1) but not in the control. (b) RT-PCR on RNA demonstrated loss of all products containing exon 1 in the affected individual but not in the control. (c) qPCR on RNA demonstrated non-expression of exon 1 the in affected individual. The homozygous deletion identified in two affected individuals from Family 1 partially deletes exon 1 of the predominant DCC isoform that is expressed in normal adult and fetal brain, X76132 (Refseq mRNA NM_005215.3).
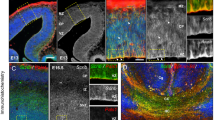
Supplementary Figure 6 Diffusion tensor tractography.
(a) Whole brain diffusion tensor tractography (DTT) fiber tract colored direction map of Family 2 (DCC) in comparison to an age and gender matched control individual and a subject with isolated agenesis of corpus callosum (ACC) (red, left right orientation; green, anterior posterior orientation; blue, superior inferior orientation), showing anterior, posterior coronal view, left and right sagittal views. (b) Whole brain fiber tract-fractional anisotropy (red/yellow scalar map) of control, DCC and ACC subject (yellow = 0 or low anisotropy, red = 1 or high anisotropy), showing anterior, posterior coronal view, left and right sagittal views.
Supplementary Figure 7 Diffusion tensor tractography.
(a,b) Diffusion Tensor Imaging (DTI) of the corpus callosum, obtained by placing a region of interest on the four midline sagittal slices; left and right hemisphere contribution to the tracts were visualized (two left hemisphere slices, two right hemisphere slices) and pseudocolored yellow and blue. A mixture of yellow and blue fibers, representing the left and right hemisphere tract reconstructions, and the presence of red fibers indicate a well-defined interhemispheric crossing in the control. In individual II:1 from Family 2, there appears to be no midline region of interest corresponding to the corpus callosum. Instead, in each hemisphere, a distinct longitudinal tract known as the Probst bundle can be visualized; each hemisphere seems to have a separate connection to the frontal, motor strip, and occipital areas, but these fibers do not extend into the contralateral hemisphere. Solid white line: genu of corpus callosum. Dashed white line: splenium of corpus callosum. Dashed orange line: anterior commissure.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Tables 1–7 and Supplementary Note (PDF 2166 kb)
Rights and permissions
About this article
Cite this article
Jamuar, S., Schmitz-Abe, K., D'Gama, A. et al. Biallelic mutations in human DCC cause developmental split-brain syndrome. Nat Genet 49, 606–612 (2017). https://doi.org/10.1038/ng.3804
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3804
This article is cited by
-
Corticolimbic DCC gene co-expression networks as predictors of impulsivity in children
Molecular Psychiatry (2022)
-
Investigating regions of shared genetic variation in attention deficit/hyperactivity disorder and major depressive disorder: a GWAS meta-analysis
Scientific Reports (2021)
-
Unique bioinformatic approach and comprehensive reanalysis improve diagnostic yield of clinical exomes
European Journal of Human Genetics (2019)
-
Reduced gene expression of netrin family members in skin and sural nerve specimens of patients with painful peripheral neuropathies
Journal of Neurology (2019)
-
Next-generation sequencing analysis of multiplex families with atypical psychosis
Translational Psychiatry (2018)