Abstract
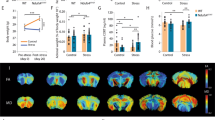
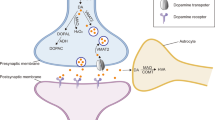
MAOA and MAOB are key iso-enzymes that degrade biogenic and dietary amines1–5. MAOA preferentially oxidizes serotonin (5-hydroxytryptamine, or 5-HT) and nore-pinephrine (NE), whereas MAOB preferentially oxidizes β-phenylethylamine (PEA). Both forms can oxidize dopamine (DA). A mutation in MAOA results in a clinical phenotype characterized by borderline mental retardation and impaired impulse control6,7. X-chromosomal deletions which include MAOB were found in patients suffering from atypical Norrie's disease8,9, which is characterized by blindness and impaired hearing. Reduced MAOB activity has been found in type-ll alcoholism and in cigarette smokers10,11. Because most alcoholics smoke, the effects of alcohol on MAOB activity remain to be determined. Here we show that targetted inactivation of MAOB in mice increases levels of PEA but not those of 5-HT, NE and DA, demonstrating a primary role for MAOB in the metabolism of PEA. PEA has been implicated in modulating mood and affect12,13. Indeed, MAOB-deficient mice showed an increased reactivity to stress. In addition, mutant mice were resistant to the neurodegenerative effects of MPTP, a toxin that induces a condition reminiscent of Parkinson's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bach, A.W. et al. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc. Natl. Acad. Sci. USA 85, 4934–4938 (1988).
Lan, N.C. et al. Human monoamine oxidase A and B genes map to Xp11.23 and are deleted in a patient with Norrie disease. Genomics 4, 552–559 (1989).
Grimsby, J., Chen, K., Wang, L.J., Lan, N.C. & Shih, J.C. Monoamine oxidase A and B genes exhibit identical exon-intron organization. Proc. Natl. Acad. Sci. USA 88, 3637–3641 (1991).
Chen, K., Wu, H.F., Grimsby, J. & Shih, J.C. Cloning of a novel monoanine oxidase cDNAfrom trout liver. Mol. Pharmacol. 46, 1226–1233 (1994).
Chen, K., Wu, H.F. & Shih, J.C. Influence of C terminus on monoamine oxidase A and B catalytic activity. J. Neurochem. 66, 797–803 (1996).
Brunner, H.G., Nelen, M.R., Breakfield, X.O., Ropers, H.H. & Oost, B.A. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 262, 578–580 (1993).
Brunner, H.G. et al. X-Linked borderline mental retardation with prominent behavioral disturbance: phenotype, genetic localization, and evidence for disturbed monoamine metabolism. Am. J. Hum. Genet. 52, 1032–1039 (1993).
Lenders, J.W.M. et al. Specific genetic deficiencies of the A and B isoenzymes of monoamine oxidase are characterized by distinct neurochemical and clinical phenotypes. J. Clin. Invest. 97, 1010–1019 (1996).
Collins, F.A. et al. Clinical, biochemical, and neuropsychiatric evaluation of a patient with a contiguous gene syndrome due to a microdeletion Xp11.3 including the Norrie disease locus and monoamine oxidase (MAOA and MAOB) genes. Am. J. Med. Genet. 42, 127–134 (1992).
Devor, E.J., Cloninger, C.R., Hoffman, P.L. & Tabakoff, B. Association of monoamine oxidase (MAO) activity with alcoholism and alcoholic sybtypes. Am. J. Med. Genet. 48, 209–213 (1993).
Fowler, J.S. et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature 379, 733–736 (1996).
Linnoila, M., Karoum, F., Cutler, N.R. & Potter, W.Z. Temporal association between depression dependent dyskinesias and high urinary pheynethylamine output. Biol. Psychiatry 18, 513–518 (1983).
Potkin, S.G., Wyatt, R.J. & Karoum, F. Psychostimulants and dopaminergic agonists in the study of adult hyperkinesis, schizophrenia and affective disorders. Psychopharmacol. Bull. 16, 52–59 (1980).
Saura, J., Richards, J.G. & Mahy, N. Differential age-related changes of MAO-A and MAO-B in mouse brain and peripheral organs. Neurobiol. Aging 15, 399–408 (1994).
Cases, O. et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science 268, 1763–1766 (1995).
Adams, J., Kalivas, P.W. & Miller, C.A. The acute histopathy of MPTP in the mouse CNS. Brain Res. Bull. 23, 1–17 (1989).
Gerlach, M., Youdim, M.B. & Riederer, P. Pharmacology of selegiline. Neurology 47, 5137–5145 (1996).
Juorio, A.V., Greenshaw, A.J. & Wishart, T.B. Reciprocal changes in striatal dopamine and β-phenylethylamine induced by reserpine in the presence of monoamine oxidase inhibitors. Naunyn Schmiedebergs Arch. Pharmacol. 338, 644–648 (1988).
Berry, M.D., Scarr, E., Zhu, M.Y., Paterson, I.A. & Juorio, A.V. The effects of administration of monamine oxidase-B inhibitors on rat striatal neurone responses to dopamine. Br. J. Pharmacol. 113, 1159–1166 (1994).
Paterson, I.A., Juorio, A.V. & Boulton, A.A. 2-Phenylethylamine: a modulator of catecholamine transmission in the mammalian central nervous system. J. Neurochem. 55, 1827–1837 (1990).
Sabelli, H.C. et al. Clinical studies on the phenylethylamine hypothesis of affective disorder: urine and blood phenylacetic acid and phenylethylamine dietary supplements. J. Clin. Psychiatry 47, 66–70 (1986).
Sano, M. et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. N. Engl. J. Med. 336, 1216–1222 (1997).
Porsolt, R.D., Bertin, A. & Jalfre, M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 229, 327–336 (1977).
Fozard, J.R., Palfreyman, M.G. & Zreika, M. The comparative pharmacology of two selective inhibitors of monoamine oxidase B: deprenyl and MDL 72145 Br. J. Pharmacol. 82, 309P (1984).
Cases, O. et al. Lack of barrels in the somatosensory cortex of monoamine oxidase-A–deficient mice: role of a serotonin excess during the critical period. Neuron 16, 297–307 (1996).
McFarland, D.J. Effects of scopolamine, D-amphetamine, and apomorphine on alternation and position biases. Pharmacol. Biochem. Behav. 32, 723–726 (1989).
Wu, H.F., Chen, K. & Shih, J.C. Site-directed mutagenesis of monoamine oxidase A and B: role of cysteines. Mol. Pharmacol. 43, 888–893 (1993).
Kumazawa, T., Suzuki, O., Seno, H. & Hattori, H. Determination of β-phenylethylamine in catfish (Parasilusus asotus) tissues by gas chromatography/mass spectrometry. Comp. Biochem. Physiol. C91, 571–574 (1988).
Dluzen, D., Reddy, A. & McDermott, J. The aromatic amino acid decarboxylase inhibitor, NSD-1015, increases release of dopamine: response characteristics. Neuropharmacology 31, 1223–1229 (1992).
Pellow, S., Chopin, P., File, S.E. & Briley, M.J. Validation of open:closed arm entries in an elevated plus maze as a measure of anxiety in the rat. J. Neurosci. Methods 14, 149–167 (1985).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grimsby, J., Toth, M., Chen, K. et al. Increased stress response and β–phenylethylamine in MAOB–deficient mice. Nat Genet 17, 206–210 (1997). https://doi.org/10.1038/ng1097-206
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1097-206
This article is cited by
-
Phenethylamine is a substrate of monoamine oxidase B in the paraventricular thalamic nucleus
Scientific Reports (2022)
-
C/EBPβ/δ-secretase signaling mediates Parkinson’s disease pathogenesis via regulating transcription and proteolytic cleavage of α-synuclein and MAOB
Molecular Psychiatry (2021)
-
Olfactory signaling via trace amine-associated receptors
Cell and Tissue Research (2021)
-
The trace aminergic system: a gender-sensitive therapeutic target for IBS?
Journal of Biomedical Science (2020)
-
Molecular docking studies of coumarin hybrids as potential acetylcholinesterase, butyrylcholinesterase, monoamine oxidase A/B and β-amyloid inhibitors for Alzheimer’s disease
Chemistry Central Journal (2018)