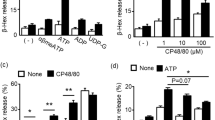
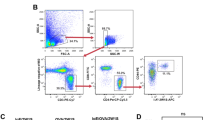
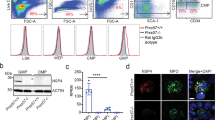
Abstract
Mast cells are key effector cells in allergic reactions. Aggregation of the receptor FcεRI in mast cells triggers the influx of calcium (Ca2+) and the release of inflammatory mediators. Here we show that transient receptor potential TRPM4 proteins acted as calcium-activated nonselective cation channels and critically determined the driving force for Ca2+ influx in mast cells. Trpm4−/− bone marrow–derived mast cells had more Ca2+ entry than did TRPM4+/+ cells after FcεRI stimulation. Consequently, Trpm4−/− bone marrow–derived mast cells had augmented degranulation and released more histamine, leukotrienes and tumor necrosis factor. Trpm4−/− mice had a more severe IgE-mediated acute passive cutaneous anaphylactic response, whereas late-phase passive cutaneous anaphylaxis was not affected. Our results establish the physiological function of TRPM4 channels as critical regulators of Ca2+ entry in mast cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Galli, S.J., Nakae, S. & Tsai, M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 6, 135–142 (2005).
Metcalfe, D.D., Baram, D. & Mekori, Y.A. Mast cells. Physiol. Rev. 77, 1033–1079 (1997).
Ali, H., Cunha-Melo, J.R., Saul, W.F. & Beaven, M.A. Activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen-stimulated RBL-2H3 cells. Evidence for a novel adenosine receptor. J. Biol. Chem. 265, 745–753 (1990).
Ramkumar, V., Stiles, G.L., Beaven, M.A. & Ali, H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J. Biol. Chem. 268, 16887–16890 (1993).
Gilfillan, A.M. & Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 6, 218–230 (2006).
Blank, U. & Rivera, J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 25, 266–273 (2004).
Jacobson, K.A. & Gao, Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 5, 247–264 (2006).
Wymann, M.P. et al. Phosphoinositide 3-kinase γ: a key modulator in inflammation and allergy. Biochem. Soc. Trans. 31, 275–280 (2003).
Ozawa, K. et al. Ca2+-dependent and Ca2+-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J. Biol. Chem. 268, 1749–1756 (1993).
Beaven, M.A., Moore, J.P., Smith, G.A., Hesketh, T.R. & Metcalfe, J.C. The calcium signal and phosphatidylinositol breakdown in 2H3 cells. J. Biol. Chem. 259, 7137–7142 (1984).
Hoth, M. & Penner, R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355, 353–356 (1992).
Mohr, F.C. & Fewtrell, C. Depolarization of rat basophilic leukemia cells inhibits calcium uptake and exocytosis. J. Cell Biol. 104, 783–792 (1987).
Mohr, F.C. & Fewtrell, C. IgE receptor-mediated depolarization of rat basophilic leukemia cells measured with the fluorescent probe bis-oxonol. J. Immunol. 138, 1564–1570 (1987).
Colquhoun, D., Neher, E., Reuter, H. & Stevens, C.F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature 294, 752–754 (1981).
Prawitt, D. et al. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i . Proc. Natl. Acad. Sci. USA 100, 15166–15171 (2003).
Launay, P. et al. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109, 397–407 (2002).
Nilius, B. et al. Voltage dependence of the Ca2+-activated cation channel TRPM4. J. Biol. Chem. 278, 30813–30820 (2003).
Ramsey, I.S., Delling, M. & Clapham, D.E. An introduction to TRP channels. Annu. Rev. Physiol. 68, 619–647 (2006).
Petersen, O.H. Cation channels: homing in on the elusive CAN channels. Curr. Biol. 12, R520–R522 (2002).
Cheng, H. et al. TRPM4 controls insulin secretion in pancreatic β-cells. Cell Calcium 41, 51–61 (2007).
Launay, P. et al. TRPM4 regulates calcium oscillations after T cell activation. Science 306, 1374–1377 (2004).
Hamill, O.P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv Eur. J. Physiol. 391, 85–100 (1981).
Hille, B. Ionic Channels of Excitable Membranes (Sinauer Associates, Sunderland, Massachusetts, 2001).
Nilius, B. et al. The selectivity filter of the cation channel TRPM4. J. Biol. Chem. 280, 22899–22906 (2005).
Salvatore, C. et al. Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J. Biol. Chem. 275, 4429–4434 (2000).
Marquardt, D., Walker, L. & Wasserman, S. Adenosine receptors on mouse bone marrow-derived mast cells: functional significance and regulation by aminophylline. J. Immunol. 133, 932–937 (1984).
Duffy, S.M., Lawley, W.J., Conley, E.C. & Bradding, P. Resting and activation-dependent ion channels in human mast cells. J. Immunol. 167, 4261–4270 (2001).
Fernandez, J.M., Neher, E. & Gomperts, B.D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. Nature 312, 453–455 (1984).
Lindau, M. & Neher, E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 411, 137–146 (1988).
Marshall, J.S. Mast-cell responses to pathogens. Nat. Rev. Immunol. 4, 787–799 (2004).
Qiao, H., Andrade, M.V., Lisboa, F.A., Morgan, K. & Beaven, M.A. FcεR1 and Toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood 107, 610–618 (2006).
Inagaki, N., Goto, S., Nagai, H. & Koda, A. Homologous passive cutaneous anaphylaxis in various strains of mice. Int. Arch. Allergy Appl. Immunol. 81, 58–62 (1986).
Klemm, S. et al. The Bcl10-Malt1 complex segregates FcεRI-mediated nuclear factor κB activation and cytokine production from mast cell degranulation. J. Exp. Med. 203, 337–347 (2006).
Wershil, B.K., Wang, Z.S., Gordon, J.R. & Galli, S.J. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-α. J. Clin. Invest. 87, 446–453 (1991).
Nilius, B. & Vennekens, R. From cardiac cation channels to the molecular dissection of the transient receptor potential channel TRPM4. Pflugers Arch. 453, 313–321 (2006).
Nilius, B. et al. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J. Biol. Chem. 280, 6423–6433 (2005).
Nilius, B. et al. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 25, 467–478 (2006).
Zhang, Z., Okawa, H., Wang, Y. & Liman, E.R. Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J. Biol. Chem. 280, 39185–39192 (2005).
Sasaki, J. et al. Regulation of anaphylactic responses by phosphatidylinositol phosphate kinase type I α. J. Exp. Med. 201, 859–870 (2005).
Duffy, S.M. et al. The K+ channel iKCA1 potentiates Ca2+ influx and degranulation in human lung mast cells. J. Allergy Clin. Immunol. 114, 66–72 (2004).
Earley, S., Waldron, B.J. & Brayden, J.E. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ. Res. 95, 922–929 (2004).
Fioretti, B., Franciolini, F. & Catacuzzeno, L. A model of intracellular Ca2+ oscillations based on the activity of the intermediate-conductance Ca2+-activated K+ channels. Biophys. Chem. 113, 17–23 (2005).
Lewis, R. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 19, 497–521 (2001).
Peters-Golden, M., Canetti, C., Mancuso, P. & Coffey, M.J. Leukotrienes: underappreciated mediators of innate immune responses. J. Immunol. 174, 589–594 (2005).
Talavera, K. et al. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438, 1022–1025 (2005).
Kaplan, A.P., Garofalo, J., Sigler, R. & Hauber, T. Idiopathic cold urticaria: in vitro demonstration of histamine release upon challenge of skin biopsies. N. Engl. J. Med. 305, 1074–1077 (1981).
Nagai, H. et al. Role of mast cells in the onset of IgE-mediated late-phase cutaneous response in mice. J. Allergy Clin. Immunol. 106, S91–S98 (2000).
AAAAI. The Allergy Report (The American Academy of Allergy, Asthma and Immunology, Milwaukee, Wisconsin, USA, 2000).
Laffargue, M. et al. Phosphoinositide 3-kinase γ is an essential amplifier of mast cell function. Immunity 16, 441–451 (2002).
Vriens, J. et al. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. USA 101, 396–401 (2004).
Yoshimura, T., Kaneuchi, T., Miura, T. & Kimura, M. Kinetic analysis of the fluorescence reaction of histamine with orthophthalaldehyde. Anal. Biochem. 164, 132–137 (1987).
Ali, K. et al. Essential role for the p110δ phosphoinositide 3-kinase in the allergic response. Nature 431, 1007–1011 (2004).
Acknowledgements
We thank S. Collins, R. Ramracheya and P. Rorsman for measuring insulin release from pancreatic islets; M. Thomas, M. Wymann, G. Opdenakker and T. Voets for discussions and for help with capacitance measurements (T.V.) and the PCA protocol (M.T. and M.W.); and J. Prenen, E. Martens, S. Buchholz, K. Fischer and C. Wesely for technical assistance. Supported by the Deutsche Forschungsgemeinschaft (M.F., S.P. and V.F.), Fonds der Chemischen Industrie and Sander-Stiftung (V.F.), Forschungsausschuss der Universität des Saarlandes (M.F., V.F.), the Human Frontiers Science Programme (RGP 32/2004 to R.V. and B.N.), the Belgian and Flemish Federal Government (GOA 2004/07, F.W.O., G.0136.00, F.W.O., G.0172.03 and IUAP Nr.3P4/23; Excellentiefinanciering EF/95/010 to R.V. and B.N.), the Flemish fund of scientific research (FWO-Vlaanderen; R.V.) and the Alexander von Humboldt-Stiftung (R.V.).
Author information
Authors and Affiliations
Contributions
R.V., M.F., B.N. and V.F. contributed to all aspects of the manuscript (conceptual design, experimentation, mouse work, writing); J.O. contributed to gene targeting; M.M. to contributed protein chemistry; F.S. and P.W. contributed to morphological characterization of BMMCs; W.B. contributed to histology, immunocytochemistry, immunohistochemistry and electron microscopy, I.M. contributed to anaphylaxis and glucose tolerance experiments; and S.E.P. performed cell-sorting and RT-PCR of B and T cells.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Targeted disruption of the Trpm4 gene. (PDF 95 kb)
Supplementary Fig. 2
Pancreatic β-cell function and expression analysis of Trpm4 and Trpm5 in Trpm4+/+ and Trpm4-/- mice. (PDF 168 kb)
Supplementary Fig. 3
Permeation properties of the endogenous Ca2+-activated cation current in BMMCs. (PDF 130 kb)
Supplementary Fig. 4
Ca2+ signaling and membrane potential in Trpm4+/+ and Trpm4-/- mast cells following DNP and combined adenosine+DNP stimulation in Ca2+-free and 156 mM K+ medium. (PDF 162 kb)
Supplementary Fig. 5
Ca2+ release–activated Ca2+ currents and FcεRI induced signaling in Trpm4+/+ and Trpm4-/- mast cells. (PDF 181 kb)
Supplementary Fig. 6
Membrane potential measurements in BMMCs after antigen stimulation. (PDF 143 kb)
Supplementary Fig. 7
Ca2+-activated Cl– and K+ currents in Trpm4+/+ and Trpm4-/- mast cells and a model for the role of TRPM4 in FcεRI-induced Ca2+ signaling. (PDF 185 kb)
Supplementary Fig. 8
GTP-γ-S-induced capacitance changes and LPS-induced activation of Trpm4+/+ and Trpm4-/- mast cells. (PDF 209 kb)
Rights and permissions
About this article
Cite this article
Vennekens, R., Olausson, J., Meissner, M. et al. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol 8, 312–320 (2007). https://doi.org/10.1038/ni1441
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1441
This article is cited by
-
TRPM channels in health and disease
Nature Reviews Nephrology (2024)
-
A glibenclamide-sensitive TRPM4-mediated component of CA1 excitatory postsynaptic potentials appears in experimental autoimmune encephalomyelitis
Scientific Reports (2022)
-
TRP channel expression correlates with the epithelial–mesenchymal transition and high-risk endometrial carcinoma
Cellular and Molecular Life Sciences (2022)
-
Development and characterization of a monoclonal antibody blocking human TRPM4 channel
Scientific Reports (2021)
-
Upregulation of transient receptor potential melastatin 4 (TRPM4) in ventricular fibroblasts from heart failure patients
Pflügers Archiv - European Journal of Physiology (2021)