Abstract
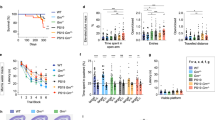
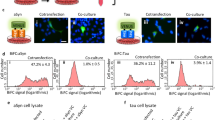
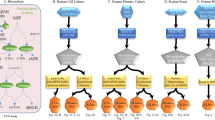
Because of their abundance, resistance to proteolysis, rapid aggregation and neurotoxicity, N-terminally truncated and, in particular, pyroglutamate (pE)-modified Aβ peptides have been suggested as being important in the initiation of pathological cascades resulting in the development of Alzheimer's disease1,2,3,4,5,6. We found that the N-terminal pE-formation is catalyzed by glutaminyl cyclase in vivo. Glutaminyl cyclase expression was upregulated in the cortices of individuals with Alzheimer's disease and correlated with the appearance of pE-modified Aβ. Oral application of a glutaminyl cyclase inhibitor resulted in reduced Aβ3(pE)–42 burden in two different transgenic mouse models of Alzheimer's disease and in a new Drosophila model. Treatment of mice was accompanied by reductions in Aβx–40/42, diminished plaque formation and gliosis and improved performance in context memory and spatial learning tests. These observations are consistent with the hypothesis that Aβ3(pE)–42 acts as a seed for Aβ aggregation by self-aggregation and co-aggregation with Aβ1–40/42. Therefore, Aβ3(pE)–40/42 peptides seem to represent Aβ forms with exceptional potency for disturbing neuronal function. The reduction of brain pE-Aβ by inhibition of glutaminyl cyclase offers a new therapeutic option for the treatment of Alzheimer's disease and provides implications for other amyloidoses, such as familial Danish dementia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hardy, J.A. & Higgins, G.A. Alzheimer's disease: the amyloid cascade hypothesis. Science 256, 184–185 (1992).
Iwatsubo, T. et al. Visualization of Aβ 42(43) and Aβ 40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ 42(43). Neuron 13, 45–53 (1994).
Iwatsubo, T., Mann, D.M., Odaka, A., Suzuki, N. & Ihara, Y. Amyloid beta protein (Aβ) deposition: Aβ 42(43) precedes Aβ 40 in Down syndrome. Ann. Neurol. 37, 294–299 (1995).
Saido, T.C. et al. Dominant and differential deposition of distinct β-amyloid peptide species, Aβ N3(pE), in senile plaques. Neuron 14, 457–466 (1995).
Russo, C. et al. Presenilin-1 mutations in Alzheimer's disease. Nature 405, 531–532 (2000).
Saido, T.C., Yamao, H., Iwatsubo, T. & Kawashima, S. Amino- and carboxyl-terminal heterogeneity of β-amyloid peptides deposited in human brain. Neurosci. Lett. 215, 173–176 (1996).
Naslund, J. et al. Relative abundance of Alzheimer Aβ amyloid peptide variants in Alzheimer disease and normal aging. Proc. Natl. Acad. Sci. USA 91, 8378–8382 (1994).
Liu, K. et al. Characterization of Aβ11–40/42 peptide deposition in Alzheimer's disease and young Down's syndrome brains: implication of N-terminally truncated Aβ species in the pathogenesis of Alzheimer's disease. Acta Neuropathol. 112, 163–174 (2006).
Russo, C. et al. PE-modified amyloid β-peptides–AβN3(pE)–strongly affect cultured neuron and astrocyte survival. J. Neurochem. 82, 1480–1489 (2002).
Saido, T.C. Alzheimer's disease as proteolytic disorders: anabolism and catabolism of β-amyloid. Neurobiol. Aging 19, S69–S75 (1998).
Schilling, S. et al. On the seeding and oligomerization of pGlu-amyloid peptides (in vitro). Biochemistry 45, 12393–12399 (2006).
Miravalle, L. et al. Amino-terminally truncated Aβ peptide species are the main component of cotton wool plaques. Biochemistry 44, 10810–10821 (2005).
Schilling, S., Hoffmann, T., Manhart, S., Hoffmann, M. & Demuth, H.-U. Glutaminyl cyclases unfold glutamyl cyclase activity under mild acid conditions. FEBS Lett. 563, 191–196 (2004).
Cynis, H. et al. Inhibition of glutaminyl cyclase alters pE formation in mammalian cells. Biochim. Biophys. Acta 1764, 1618–1625 (2006).
Pohl, T., Zimmer, M., Mugele, K. & Spiess, J. Primary structure and functional expression of a glutaminyl cyclase. Proc. Natl. Acad. Sci. USA 88, 10059–10063 (1991).
Sykes, P.A., Watson, S.J., Temple, J.S. & Bateman, R.C.J. Evidence for tissue-specific forms of glutaminyl cyclase. FEBS Lett. 455, 159–161 (1999).
Buchholz, M. et al. The first potent inhibitors for human glutaminyl cyclase: synthesis and structure-activity relationship. J. Med. Chem. 49, 664–677 (2006).
Jacobsen, J.S. et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA 103, 5161–5166 (2006).
Piccini, A. et al. β-amyloid is different in normal aging and in Alzheimer disease. J. Biol. Chem. 280, 34186–34192 (2005).
Pike, C.J., Overman, M.J. & Cotman, C.W. Amino-terminal deletions enhance aggregation of β-amyloid peptides in vitro. J. Biol. Chem. 270, 23895–23898 (1995).
Vanderstichele, H. et al. Amino-truncated β-amyloid42 peptides in cerebrospinal fluid and prediction of progression of mild cognitive impairment. Clin. Chem. 51, 1650–1660 (2005).
Hashimoto, T. et al. CLAC: a novel Alzheimer amyloid plaque component derived from a transmembrane precursor, CLAC-P/collagen type XXV. EMBO J. 21, 1524–1534 (2002).
Yu, L. et al. Investigation of N-terminal glutamate cyclization of recombinant monoclonal antibody in formulation development. J. Pharm. Biomed. Anal. 42, 455–463 (2006).
Schilling, S. et al. Inhibition of glutaminyl cyclase prevents pGlu-Aβ formation after intracortical/hippocampal microinjection in vivo/in situ. J. Neurochem. 106, 1225–1236 (2008).
Kawarabayashi, T. et al. Age-dependent changes in brain, CSF, and plasma amyloid (β) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J. Neurosci. 21, 372–381 (2001).
Rockenstein, E., Mallory, M., Mante, M., Sisk, A. & Masliah, E. Early formation of mature amyloid-β protein deposits in a mutant APP transgenic model depends on levels of Aβ (1–42). J. Neurosci. Res. 66, 573–582 (2001).
Ghiso, J. et al. Chromosome 13 dementia syndromes as models of neurodegeneration. Amyloid 8, 277–284 (2001).
Tomidokoro, Y. et al. Familial Danish dementia: co-existence of Danish and Alzheimer amyloid subunits (ADan AND Aβ) in the absence of compact plaques. J. Biol. Chem. 280, 36883–36894 (2005).
Shirotani, K., Tsubuki, S., Lee, H.J., Maruyama, K. & Saido, T.C. Generation of amyloid beta peptide with pE at position 3 in primary cortical neurons. Neurosci. Lett. 327, 25–28 (2002).
Hutter-Paier, B. et al. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer's disease. Neuron 44, 227–238 (2004).
Acknowledgements
We thank K. Sowa, R. Jendrek, K. Schulz, M. Bornack, E. Scheel and M. Buchholz for technical assistance. The authors would like to express their gratitude to K. Hsiao (University of Minnesota) for kindly providing Tg2576 mice and E. Masliah (University of California, San Diego) for providing the TASD-41 mice. Thanks are due to T.C. Saido (Riken Brain Science Institute) for the gift of the APP-NLE vector. S. Winter maintained and analyzed organotypic brain slice cultures from Tg2576 mice. We are also grateful to J. Heins for statistical analysis and to M.T. Stubbs, K. Glund and M. Hartlage-Rübsamen for helpful discussion. This work was supported by German Federal Department of Education, Science and Technology BMBF grant #3013185 to H.-U.D.
Author information
Authors and Affiliations
Contributions
T.H., S.S. and S.R. planned most of the experiments. S.S., H.C., T.H., A.K., D.S. and U.H. conducted most of the biochemical and cell biological investigations. S.S., A.K., W.J., M.F. and M.H. analyzed the human brain tissue. U.Z. and S.R. carried out the Tg2576 mouse experiments. M.P., B.H.-P. and M.W. performed the TASD-41 mouse experiments. D.M. conducted the behavioral analysis of Tg2576. C.L., T.R. and G.R. generated the transgenic Drosophila lines. S.R., S.S. and H.-U.D. designed the study and wrote the manuscript. H.-U.D. initiated the research and supervised the program.
Corresponding author
Ethics declarations
Competing interests
S.S., T.H., U.H., A.K., W.J., D.S., H.C. and H.-U.D. are employees of Probiodrug AG or Ingenium GmbH, a privately held biotechnology company in Germany. H.-U.D. holds shares in the united company. B.H.-P., M.P. and M.W. are employees of JSW-CRS Research, a privately owned contract research organization in Austria. M.W. holds stock in the company.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Methods (PDF 1850 kb)
Rights and permissions
About this article
Cite this article
Schilling, S., Zeitschel, U., Hoffmann, T. et al. Glutaminyl cyclase inhibition attenuates pyroglutamate Aβ and Alzheimer's disease–like pathology. Nat Med 14, 1106–1111 (2008). https://doi.org/10.1038/nm.1872
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1872
This article is cited by
-
Peripheral administration of nanomicelle-encapsulated anti-Aβ oligomer fragment antibody reduces various toxic Aβ species in the brain
Journal of Nanobiotechnology (2023)
-
Solid-phase synthesis and pathological evaluation of pyroglutamate amyloid-β3-42 peptide
Scientific Reports (2023)
-
The E3 ligase adapter cereblon targets the C-terminal cyclic imide degron
Nature (2022)
-
Amyloid β and tau pathology in brains of aged pinniped species (sea lion, seal, and walrus)
Acta Neuropathologica Communications (2021)
-
Neuroplastin expression is essential for hearing and hair cell PMCA expression
Brain Structure and Function (2021)