Abstract
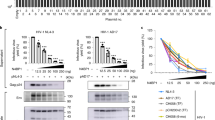
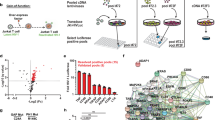
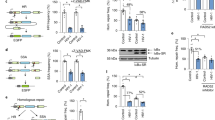
Long-standing evidence indicates that quiescent human peripheral blood T lymphocytes (PBLs) do not support efficient HIV infection. In resting PBLs, reverse transcription of viral RNA takes longer than in activated cells1, partially because formation of the late products of reverse transcription is decreased by RNA binding by apolipoprotein B mRNA–editing enzyme, catalytic polypeptide-like 3G (APOBEC3G)2. In a subsequent step, integration of the viral complementary DNA that is eventually formed is markedly impaired3,4. Here we show that cellular c-Jun N-terminal kinase (JNK), an enzyme that is not expressed in resting CD4+ T cells, regulates permissiveness to HIV-1 infection, and we unravel a new, sequential post-translational pathway of protein modification that regulates viral DNA integration. We found that, in activated T lymphocytes, viral integrase, which mediates HIV-1 cDNA integration into the host cell genome, is phosphorylated by JNK on a highly conserved serine residue in its core domain. Phosphorylated integrase, in turn, becomes a substrate for the cellular peptidyl prolyl-isomerase enzyme Pin1, which catalyzes a conformational modification of integrase. These concerted activities increase integrase stability and are required for efficient HIV-1 integration and infection. Lack of these modifications restricts viral infection in nonactivated, primary CD4+ T lymphocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zhou, Y., Zhang, H., Siliciano, J.D. & Siliciano, R.F. Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells. J. Virol. 79, 2199–2210 (2005).
Chiu, Y.L. et al. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature 435, 108–114 (2005).
Zack, J.A. et al. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61, 213–222 (1990).
Stevenson, M., Stanwick, T.L., Dempsey, M.P. & Lamonica, C.A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9, 1551–1560 (1990).
Weiss, A. & Littman, D.R. Signal transduction by lymphocyte antigen receptors. Cell 76, 263–274 (1994).
Rincón, M., Flavell, R.A. & Davis, R.J. Signal transduction by MAP kinases in T lymphocytes. Oncogene 20, 2490–2497 (2001).
Weiss, L. et al. Regulation of c-Jun NH2-terminal kinase (Jnk) gene expression during T cell activation. J. Exp. Med. 191, 139–146 (2000).
Cereseto, A. et al. Acetylation of HIV-1 integrase by p300 regulates viral integration. EMBO J. 24, 3070–3081 (2005).
Pierson, T.C. et al. Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J. Virol. 76, 8518–8531 (2002).
Suzuki, Y. & Craigie, R. The road to chromatin—nuclear entry of retroviruses. Nat. Rev. Microbiol. 5, 187–196 (2007).
Wiskerchen, M. & Muesing, M.A. Human immunodeficiency virus type 1 integrase: effects of mutations on viral ability to integrate, direct viral gene expression from unintegrated viral DNA templates and sustain viral propagation in primary cells. J. Virol. 69, 376–386 (1995).
Engelman, A., Englund, G., Orenstein, J.M., Martin, M.A. & Craigie, R. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J. Virol. 69, 2729–2736 (1995).
Whitehurst, C.E., Boulton, T.G., Cobb, M.H. & Geppert, T.D. Extracellular signal-regulated kinases in T cells. Anti-CD3 and 4 β-phorbol 12-myristate 13-acetate-induced phosphorylation and activation. J. Immunol. 148, 3230–3237 (1992).
Paolinelli, R., Mendoza-Maldonado, R., Cereseto, A. & Giacca, M. Acetylation by GCN5 regulates CDC6 phosphorylation in the S phase of the cell cycle. Nat. Struct. Mol. Biol. 16, 412–420 (2009).
Lu, K.P. & Zhou, X.Z. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat. Rev. Mol. Cell Biol. 8, 904–916 (2007).
Zhou, X.Z. et al. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol. Cell 6, 873–883 (2000).
Stukenberg, P.T. & Kirschner, M.W. Pin1 acts catalytically to promote a conformational change in Cdc25. Mol. Cell 7, 1071–1083 (2001).
Mantovani, F. et al. Pin1 links the activities of c-Abl and p300 in regulating p73 function. Mol. Cell 14, 625–636 (2004).
Zacchi, P. et al. The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature 419, 853–857 (2002).
Ryo, A. et al. Regulation of NF-κB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell 12, 1413–1426 (2003).
Ryo, A., Nakamura, M., Wulf, G., Liou, Y.C. & Lu, K.P. Pin1 regulates turnover and subcellular localization of β-catenin by inhibiting its interaction with APC. Nat. Cell Biol. 3, 793–801 (2001).
Uchida, T. et al. Pin1 and Par14 peptidyl prolyl isomerase inhibitors block cell proliferation. Chem. Biol. 10, 15–24 (2003).
Llano, M., Delgado, S., Vanegas, M. & Poeschla, E.M. Lens epithelium-derived growth factor/p75 prevents proteasomal degradation of HIV-1 integrase. J. Biol. Chem. 279, 55570–55577 (2004).
Mousnier, A. et al. von Hippel Lindau binding protein 1–mediated degradation of integrase affects HIV-1 gene expression at a postintegration step. Proc. Natl. Acad. Sci. USA 104, 13615–13620 (2007).
Bukrinsky, M.I., Stanwick, T.L., Dempsey, M.P. & Stevenson, M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science 254, 423–427 (1991).
Chun, T.W. et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387, 183–188 (1997).
Eckstein, D.A. et al. HIV-1 actively replicates in naive CD4+ T cells residing within human lymphoid tissues. Immunity 15, 671–682 (2001).
Zhang, Z. et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286, 1353–1357 (1999).
Kreisberg, J.F., Yonemoto, W. & Greene, W.C. Endogenous factors enhance HIV infection of tissue naive CD4 T cells by stimulating high molecular mass APOBEC3G complex formation. J. Exp. Med. 203, 865–870 (2006).
Yamashita, M. & Emerman, M. Retroviral infection of non-dividing cells: old and new perspectives. Virology 344, 88–93 (2006).
Zhang, J., Scadden, D.T. & Crumpacker, C.S. Primitive hematopoietic cells resist HIV-1 infection via p21. J. Clin. Invest. 117, 473–481 (2007).
Asada, M. et al. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J. 18, 1223–1234 (1999).
Schwartz, O., Marechal, V., Friguet, B., Arenzana-Seisdedos, F. & Heard, J.M. Antiviral activity of the proteasome on incoming human immunodeficiency virus type 1. J. Virol. 72, 3845–3850 (1998).
Santoni de Sio, F.R., Cascio, P., Zingale, A., Gasparini, M. & Naldini, L. Proteasome activity restricts lentiviral gene transfer into hematopoietic stem cells and is down-regulated by cytokines that enhance transduction. Blood 107, 4257–4265 (2006).
Repnik, U., Knezevic, M. & Jeras, M. Simple and cost-effective isolation of monocytes from buffy coats. J. Immunol. Methods 278, 283–292 (2003).
Tan, W., Zhu, K., Segal, D.J., Barbas, C.F. III & Chow, S.A. Fusion proteins consisting of human immunodeficiency virus type 1 integrase and the designed polydactyl zinc finger protein E2C direct integration of viral DNA into specific sites. J. Virol. 78, 1301–1313 (2004).
Butler, S.L., Hansen, M.S. & Bushman, F.D. A quantitative assay for HIV DNA integration in vivo. Nat. Med. 7, 631–634 (2001).
Todorovic, V. et al. Human origins of DNA replication selected from a library of nascent DNA. Mol. Cell 19, 567–575 (2005).
Marzio, G., Tyagi, M., Gutierrez, M.I. & Giacca, M. HIV-1 Tat transactivator recruits p300 and CREB-binding protein histone acetyltransferases to the viral promoter. Proc. Natl. Acad. Sci. USA 95, 13519–13524 (1998).
Acknowledgements
We wish to thank A. Engelman (Dana-Farber Cancer Institute) for the pFlag-IN codon-optimized expression vector, L. Collavin (Laboratorio Nazionale CIB and University of Trieste) for the p-Cs2-JNK plasmid and F. Kirchhoff (Institute of Molecular Virology, Universitätsklinikum Ulm) for the HIV-2-Rod Luc clone. We are grateful to S. Kerbavcic for excellent editorial assistance and to M. Sturnega for the technical support in raising the rabbit phospho-integrase–specific antibody. This work was supported by grants from the Italian National Research Programme on AIDS of the Istituto Superiore di Sanità, Italy to M.G. and from the Associazione Italiana per la Ricerca sul Cancro to G.D.S.
Author information
Authors and Affiliations
Contributions
L.M. conducted most of the experiments on integrase phosphorylation, Pin1 binding and HIV-1 infection; M.L. contributed to the HIV-1 infection studies and supervised part of the experiments; M.I.G. conducted the experiments on integrase stability, characterization of the phospho-integrase–specific antibody and in vitro integrase enzymatic activity; A.C. contributed to the integrase acetylation and phosphorylation studies; G.D.S supervised the integrase-Pin1 interaction studies; M.G. supervised the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 2139 kb)
Rights and permissions
About this article
Cite this article
Manganaro, L., Lusic, M., Gutierrez, M. et al. Concerted action of cellular JNK and Pin1 restricts HIV-1 genome integration to activated CD4+ T lymphocytes. Nat Med 16, 329–333 (2010). https://doi.org/10.1038/nm.2102
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2102
This article is cited by
-
A proteomic screen of Ty1 integrase partners identifies the protein kinase CK2 as a regulator of Ty1 retrotransposition
Mobile DNA (2022)
-
Activity-dependent isomerization of Kv4.2 by Pin1 regulates cognitive flexibility
Nature Communications (2020)
-
RUNX2-modifying enzymes: therapeutic targets for bone diseases
Experimental & Molecular Medicine (2020)
-
Noncovalent SUMO-interaction motifs in HIV integrase play important roles in SUMOylation, cofactor binding, and virus replication
Virology Journal (2019)
-
Cellular TRIM33 restrains HIV-1 infection by targeting viral integrase for proteasomal degradation
Nature Communications (2019)