Abstract
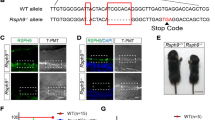
Hydrocephalus is a common neurological disorder that leads to expansion of the cerebral ventricles and is associated with a high rate of morbidity and mortality. Most neonatal cases are of unknown etiology and are likely to have complex inheritance involving multiple genes and environmental factors. Identifying molecular mechanisms for neonatal hydrocephalus and developing noninvasive treatment modalities are high priorities. Here we use a hydrocephalic mouse model of the human ciliopathy Bardet-Biedl Syndrome (BBS) and identify a role for neural progenitors in the pathogenesis of neonatal hydrocephalus. We found that hydrocephalus in this mouse model is caused by aberrant platelet-derived growth factor receptor α (PDGFR-α) signaling, resulting in increased apoptosis and impaired proliferation of chondroitin sulfate proteoglycan 4 (also known as neuron-glial antigen 2 or NG2)+PDGFR-α+ neural progenitors. Targeting this pathway with lithium treatment rescued NG2+PDGFR-α+ progenitor cell proliferation in BBS mutant mice, reducing their ventricular volume. Our findings demonstrate that neural progenitors are crucial in the pathogenesis of neonatal hydrocephalus, and we identify new therapeutic targets for this common neurological disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bruni, J.E., Del Bigio, M.R. & Clattenburg, R.E. Ependyma: normal and pathological. A review of the literature. Brain Res. 356, 1–19 (1985).
Del Bigio, M.R. Ependymal cells: biology and pathology. Acta Neuropathol. 119, 55–73 (2010).
Williams, M.A. et al. Priorities for hydrocephalus research: report from a National Institutes of Health–sponsored workshop. J. Neurosurg. 107, 345–357 (2007).
Vogel, P. et al. Congenital hydrocephalus in genetically engineered mice. Vet. Pathol. 49, 166–181 (2012).
Simon, T.D. et al. Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J. Neurosurg. Pediatr. 1, 131–137 (2008).
Shannon, C.N. et al. The economic impact of ventriculoperitoneal shunt failure. Journal J. Neurosurg. Pediatr. 8, 593–599 (2011).
Van Camp, G. et al. A duplication in the L1CAM gene associated with X-linked hydrocephalus. Nat. Genet. 4, 421–425 (1993).
Chi, J.H., Fullerton, H.J. & Gupta, N. Time trends and demographics of deaths from congenital hydrocephalus in children in the United States: National Center for Health Statistics data, 1979 to 1998. J. Neurosurg. 103, 113–118 (2005).
Zhang, J., Williams, M.A. & Rigamonti, D. Genetics of human hydrocephalus. J. Neurol. 253, 1255–1266 (2006).
Banizs, B. et al. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 132, 5329–5339 (2005).
Patwardhan, R.V. & Nanda, A. Implanted ventricular shunts in the United States: the billion-dollar-a-year cost of hydrocephalus treatment. Neurosurgery 56, 139–144 (2005).
Vogel, T.W., Carter, C.S., Abode-Iyamah, K., Zhang, Q. & Robinson, S. The role of primary cilia in the pathophysiology of neural tube defects. Neurosurg. Focus 33, E2 (2012).
Spassky, N. et al. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 25, 10–18 (2005).
Tissir, F. et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat. Neurosci. 13, 700–707 (2010).
Talos, F. et al. p73 is an essential regulator of neural stem cell maintenance in embryonal and adult CNS neurogenesis. Cell Death Differ. 17, 1816–1829 (2010).
Yang, A. et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 404, 99–103 (2000).
Drake, J.M. The surgical management of pediatric hydrocephalus. Neurosurgery 62 (suppl. 2), 633–640 (2008).
Drake, J.M., Kestle, J.R. & Tuli, S. CSF shunts 50 years on—past, present and future. Childs Nerv. Syst. 16, 800–804 (2000).
Davis, R.E. et al. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc. Natl. Acad. Sci. USA 104, 19422–19427 (2007).
Ibañez-Tallon, I. et al. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum. Mol. Genet. 13, 2133–2141 (2004).
Lancaster, M.A., Schroth, J. & Gleeson, J.G. Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nat. Cell Biol. 13, 700–707 (2011).
Ocbina, P.J., Eggenschwiler, J.T., Moskowitz, I. & Anderson, K.V. Complex interactions between genes controlling trafficking in primary cilia. Nat. Genet. 43, 547–553 (2011).
Zhang, Q., Seo, S., Bugge, K., Stone, E.M. & Sheffield, V.C. BBS proteins interact genetically with the IFT pathway to influence SHH-related phenotypes. Hum. Mol. Genet. 21, 1945–1953 (2012).
Schneider, L. et al. PDGFRαα signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 15, 1861–1866 (2005).
Kriegstein, A. & Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149–184 (2009).
Han, Y.G. et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci. 11, 277–284 (2008).
Breunig, J.J. et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc. Natl. Acad. Sci. USA 105, 13127–13132 (2008).
Sawamoto, K. et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science 311, 629–632 (2006).
Ihrie, R.A. & Alvarez-Buylla, A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron 70, 674–686 (2011).
Nachury, M.V. et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201–1213 (2007).
Baker, K. et al. Neocortical and hippocampal volume loss in a human ciliopathy: a quantitative MRI study in Bardet-Biedl syndrome. Am. J. Med. Genet. A. 155A, 1–8 (2011).
Keppler-Noreuil, K.M. et al. Brain tissue- and region-specific abnormalities on volumetric MRI scans in 21 patients with Bardet-Biedl syndrome (BBS). BMC Med. Genet. 12, 101 (2011).
von Bohlen Und Halbach, O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 329, 409–420 (2007).
Raponi, E. et al. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia 55, 165–177 (2007).
Jackson, E.L. et al. PDGFR-α+ B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron 51, 187–199 (2006).
Rivers, L.E. et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 11, 1392–1401 (2008).
Richardson, W.D., Young, K.M., Tripathi, R.B. & McKenzie, I. NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron 70, 661–673 (2011).
Tripathi, R.B., Rivers, L.E., Young, K.M., Jamen, F. & Richardson, W.D. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J. Neurosci. 30, 16383–16390 (2010).
Nishiyama, A., Komitova, M., Suzuki, R. & Zhu, X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 10, 9–22 (2009).
Kondo, T. & Raff, M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science 289, 1754–1757 (2000).
Datta, S.R. et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91, 231–241 (1997).
Vivanco, I. & Sawyers, C.L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2, 489–501 (2002).
Scheid, M.P. & Woodgett, J.R. PKB/AKT: functional insights from genetic models. Nat. Rev. Mol. Cell Biol. 2, 760–768 (2001).
Chalecka-Franaszek, E. & Chuang, D.M. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc. Natl. Acad. Sci. USA 96, 8745–8750 (1999).
Su, H., Chu, T.H. & Wu, W. Lithium enhances proliferation and neuronal differentiation of neural progenitor cells in vitro and after transplantation into the adult rat spinal cord. Exp. Neurol. 206, 296–307 (2007).
Li, H. et al. Lithium-mediated long-term neuroprotection in neonatal rat hypoxia-ischemia is associated with antiinflammatory effects and enhanced proliferation and survival of neural stem/progenitor cells. J. Cereb. Blood Flow Metab. 31, 2106–2115 (2011).
Lechtreck, K.F., Delmotte, P., Robinson, M.L., Sanderson, M.J. & Witman, G.B. Mutations in Hydin impair ciliary motility in mice. J. Cell Biol. 180, 633–643 (2008).
Goto, J., Tezuka, T., Nakazawa, T., Sagara, H. & Yamamoto, T. Loss of Fyn tyrosine kinase on the C57BL/6 genetic background causes hydrocephalus with defects in oligodendrocyte development. Mol. Cell. Neurosci. 38, 203–212 (2008).
Qin, S., Liu, M., Niu, W. & Zhang, C.L. Dysregulation of Kruppel-like factor 4 during brain development leads to hydrocephalus in mice. Proc. Natl. Acad. Sci. USA 108, 21117–21121 (2011).
Yung, Y.C. et al. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci. Transl. Med. 3, 99ra87 (2011).
Rivière, J.B. et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat. Genet. 44, 934–940 (2012).
Azim, K. & Butt, A.M. GSK3β negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia 59, 540–553 (2011).
Berbari, N.F., Lewis, J.S., Bishop, G.A., Askwith, C.C. & Mykytyn, K. Bardet-Biedl syndrome proteins are required for the localization of G protein–coupled receptors to primary cilia. Proc. Natl. Acad. Sci. USA 105, 4242–4246 (2008).
Seo, S. et al. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum. Mol. Genet. 18, 1323–1331 (2009).
Acknowledgements
We thank L. Biesecker for help obtaining the human MRI scans. We thank K. Rahmouni and D.-F. Guo for help with quantitative RT-PCR and infusion experiments. We thank K. Agassandian for help with dye injection and CSF collection. We thank V. Buffard and L. Qian for their excellent technical assistance. We also appreciate valuable assistance from the University of Iowa Central Microscopy Research Facility. This work was supported in part by US National Institutes of Health grants R01EY110298 and R01EY017168 (to V.C.S.), R01EY022616 (to S.S.), the Knight Templar Eye Foundation (to S.S.) and the Neurosurgery Research and Education Foundation (to T.W.V.). C.S.C. is a National Science Foundation graduate research fellow, and V.C.S. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
C.S.C., T.W.V. and Q.Z. conceived of the project, designed and performed experiments, coordinated collaborations and wrote the manuscript. S.S. contributed to the experimental design and manuscript revisions. R.E.S. and M.D.C. performed transmission electron microscopy, CSF collection and dye injection experiments and revised the manuscript. T.O.M. coordinated microscopic experiments. K.M.K.-N. and P.N. provided and analyzed human MRI scans. D.R.T. performed MRI for all mice. D.Y.N. and C.C.S. designed and developed the Bbs1 mouse model used in this experiment. K.B. coordinated mouse genotyping and mating. V.C.S. initiated the project, contributed ideas, analyzed and interpreted the results and helped write the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 29853 kb)
Rights and permissions
About this article
Cite this article
Carter, C., Vogel, T., Zhang, Q. et al. Abnormal development of NG2+PDGFR-α+ neural progenitor cells leads to neonatal hydrocephalus in a ciliopathy mouse model. Nat Med 18, 1797–1804 (2012). https://doi.org/10.1038/nm.2996
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2996
This article is cited by
-
BBS4 protein has basal body/ciliary localization in sensory organs but extra-ciliary localization in oligodendrocytes during human development
Cell and Tissue Research (2021)
-
Ependymal ciliary motion and their role in congenital hydrocephalus
Child's Nervous System (2021)
-
BBS4 Is Essential for Nuclear Transport of Transcription Factors Mediating Neuronal ER Stress Response
Molecular Neurobiology (2021)
-
Fyn depletion ameliorates tauP301L-induced neuropathology
Acta Neuropathologica Communications (2020)
-
The absence of BBSome function decreases synaptogenesis and causes ectopic synapse formation in the retina
Scientific Reports (2020)