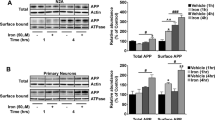
Abstract
Paired helical filament (PHF) tau is the principal component of neurofibriliary tangles, a characteristic feature of the neurodegenerative pathology in Alzheimer's disease (AD). Post-translational modification of tau, especially phosphorylation, has been considered a major factor in aggregation and diminished microtubule interactions of PHF-tau. Recently, it has been recognized that PHF-tau is also subject to non-enzymatic glycation, with formation of advanced glycation end products (AGEs). We now show that as a consequence of glycation, PHF-tau from AD and AGE-tau generate oxygen free radicals, thereby activating transcription via nuclear factor-κB, increasing amyloid β-protein precursor and release of ∼4 kD amyloid β-peptides. These data provide insight into how PHF-tau disturbs neuronal function, and add to a growing body of evidence that oxidant stress contributes to the pathogenesis of AD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Wischik, C. Cell biology of the Alzheimer tangle. Curr. Opin. Cell Biol. 1, 115–122 (1989).
Kosik, K. Alzheimer's disease sphinx: A riddle with plaques and tangles. J. Cell Biol. 127, 1501–1504 (1994).
Goedert, M. Tau protein and the neurofibrillary pathology of Alzheimer's disease. Trends Neurosci. 16, 460–465 (1993).
Trojanowski, J. & Lee, V. Paired helical filament tau in Alzheimer's disease, the kinase connection. Am. J. Pathol. 144, 449–453 (1994).
Haass, C. & Selkoe, D. Cellular processing of β-amyloid precursor protein and the genesis of amyloid β-peptide. Cell 75, 1039–1042 (1994).
Ruderman, N., Williamson, J. & Brownlee, M. Glucose and diabetic vascular disease. FASEB J. 6, 2905–2914 (1992).
Baynes, J. Role of oxidative stress in development of complications in diabetes. Diabetes 40, 405–412 (1991).
Sell, D. & Monnier, V. Structure elucidation of senescence cross-link from human extracellular matrix: implication of pentoses in the aging process. J. biol. Chem. 264, 21597–21602 (1989).
Yan, S.-D. et al. The presence of glycated tau in Alzheimer's disease: a mechanism for induction of oxidant stress. Proc. natn. Acad. Sci. U.S.A. 91, 7787–7791 (1994).
Ledesma, M., Bonay, P., Colaco, C. & Avila, J. Analysis of microtubule-associated protein tau glycation in paired helical filaments. J. biol. Chem. 269, 21614–21619 (1994).
Strauss, S. et al. Detection of interleukin-6 and α2-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer's disease patients. Lab. Invest. 66, 223–230 (1992).
Finkelstein, E., Rosen, G.M. & Rauckman, E.J. Spin trapping of superoxide and hydroxyl radical. Molec. Pharmacol. 21, 262–265 (1982).
Zweier, J., Kuppusamy, P. & Lutty, G. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in the postischemic heart. Proc. natn. Acad. Sci. U.S.A. 85, 4046–4050 (1988).
Knops, J. et al. Overexpression of tau in a nonneuronal cell induces long cellular processes. J. Cell Biol. 114, 725–733 (1991).
Frappier, T., Georgieff, I., Brown, K. & Shelanski, M. Tau regulation of microtubule spacing and bundling. J. Neurochem. 63, 2288–2294 (1994).
Schreck, R., Rieber, P. & Baeuerle, P. Reactive oxygen intermediates as apparently widely used messengers in the action of the NF-κB transcription factor and HIV-1. EMBO J. 10, 2247–2258 (1991).
Griffin, W. et al. Brain interleukin 1 and S100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. natn. Acad. Sci. U.S.A. 86, 7611–7615 (1989).
Ray, A., Tatter, S., May, L. & Sehgal, P. Activation of the human IL-6 promoter by cytokines, viruses and second messenger agonists. Proc. natn. Acad. Sci. U.S.A. 85, 6701–6705 (1988).
Shoji, M. et al. Production of Alzheimer amyloid β protein by normal proteolytic processing. Science 258, 126–129 (1992).
Loo, D. et al. Apoptosis is induced by β-amyloid in cultured central nervous system neurons. Proc. natn. Acad. Sci. U.S.A. 90, 7951–7955 (1993).
Vitek, M. et al. Advanced glycation endproducts contribute to amyloidosis in Alzheimer's disease. Proc. natn. Acad. Sci. U.S.A. 91, 4766–4770 (1994).
Smith, T. et al. Advanced maillard reaction endproducts are associated with Alzheimer disease pathology. Proc. natn. Acad. Sci. U.S.A. 91, 5710–5714 (1994).
Behl, C., Davis, J., Lesley, R. & Schubert, D. Hydrogen peroxide mediates amyloid β-protein toxicity. Cell 77, 817–827 (1994).
Collins, T. Endothelial nuclear factor κB and the initiation of the atherosclerotic lesion. Lab. Invest. 68, 499–508 (1993).
Liu, W.-K., Ksiezak-Reding, H. & Yen, S.-H. Abnormal tau proteins from Alzheimer's disease brains. J. biol. Chem. 266, 21723–21727 (1991).
Lin, M.-F., DaVolio, J. & Garcia, R. Cationic liposome-mediated incorporation of prostatic acid phosphatase protein in human prostate carcinoma cells. Biochem. biophys. Res. Commun. 192, 413–419 (1993).
Yan, S.-D. et al. Enhanced cellular oxidant stress by the interaction of advanced glycation endproducts with their receptors/binding proteins. J. biol. Chem. 269, 9882–9888 (1994).
Yavin, E. & Yavin, Z. Attachment and culture of dissociated cells from rat embryo cerebral hemispheres on polylysine-coated surfaces. J. Cell Biol. 62, 540–546 (1974).
Sakurai, T. & Tsuchiya, S. Superoxide production from nonenzymatically glycated protein. FEBS Lett. 236, 406–410 (1988).
Dignam, J., Lebovitz, R. & Roeder, R. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11, 1475–1489 (1983).
Sambrook, J., Fritsch, E. & Maniatis, T. Molecular Cloning: A Laboratory Manual 2nd edn. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1989).
Caporaso, G., Gandy, S., Buxbaum, J. & Greengard, P. Chloroquine inhibits intracellular degradation but not secretion of Alzheimer β/A4 amyloid precursor protein. Proc. natn. Acad. Sci. U.S.A. 89, 2252–2256 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yan, S., Yan, S., Chen, X. et al. Non-enzymatically glycated tau in Alzheimer's disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid β-peptide. Nat Med 1, 693–699 (1995). https://doi.org/10.1038/nm0795-693
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0795-693
This article is cited by
-
Effects of Exercise Training and L-Arginine Loaded Chitosan Nanoparticles on Hippocampus Histopathology, β-Secretase Enzyme Function, APP, Tau, Iba1and APOE-4 mRNA in Aging Rats
Neurotoxicity Research (2024)
-
Regulation of Neurodegeneration-associated Protein Fragments by the N-degron Pathways
Neurotoxicity Research (2022)
-
Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease
Molecular Neurodegeneration (2020)
-
Stress-sensing in the human greying hair follicle: Ataxia Telangiectasia Mutated (ATM) depletion in hair bulb melanocytes in canities-prone scalp
Scientific Reports (2020)
-
Alpha-linolenic acid regulates amyloid precursor protein processing by mitogen-activated protein kinase pathway and neuronal apoptosis in amyloid beta-induced SH-SY5Y neuronal cells
Applied Biological Chemistry (2018)