Abstract
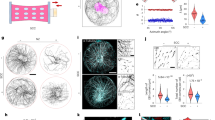
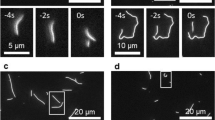
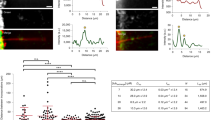
Microtubules—which define the shape of axons, cilia and flagella, and provide tracks for intracellular transport—can be highly bent by intracellular forces, and microtubule structure and stiffness are thought to be affected by physical constraints. Yet how microtubules tolerate the vast forces exerted on them remains unknown. Here, by using a microfluidic device, we show that microtubule stiffness decreases incrementally with each cycle of bending and release. Similar to other cases of material fatigue, the concentration of mechanical stresses on pre-existing defects in the microtubule lattice is responsible for the generation of more extensive damage, which further decreases microtubule stiffness. Strikingly, damaged microtubules were able to incorporate new tubulin dimers into their lattice and recover their initial stiffness. Our findings demonstrate that microtubules are ductile materials with self-healing properties, that their dynamics does not exclusively occur at their ends, and that their lattice plasticity enables the microtubules’ adaptation to mechanical stresses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mimori-Kiyosue, Y. Shaping microtubules into diverse patterns: Molecular connections for setting up both ends. Cytoskeleton 68, 603–618 (2011).
Van der Vaart, B., Akhmanova, A. & Straube, A. Regulation of microtubule dynamic instability. Biochem. Soc. Trans. 37, 1007–1013 (2009).
Schek, H. T., Gardner, M. K., Cheng, J., Odde, D. J. & Hunt, A. J. Microtubule assembly dynamics at the nanoscale. Curr. Biol. 17, 1445–1455 (2007).
Dogterom, M. & Surrey, T. Microtubule organization in vitro. Curr. Opin. Cell Biol. 25, 23–29 (2013).
Vignaud, T., Blanchoin, L. & Théry, M. Directed cytoskeleton self-organization. Trends Cell Biol. 22, 671–682 (2012).
Howard, J. Elastic and damping forces generated by confined arrays of dynamic microtubules. Phys. Biol. 3, 54–66 (2006).
Hawkins, T., Mirigian, M., Selcuk Yasar, M. & Ross, J. L. Mechanics of microtubules. J. Biomech. 43, 23–30 (2010).
Mohrbach, H., Johner, A. & Kulić, I. M. Cooperative lattice dynamics and anomalous fluctuations of microtubules. Eur. Biophys. J. 41, 217–239 (2012).
Gittes, F., Mickey, B., Nettleton, J. & Howard, J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Biol. 120, 923–934 (1993).
Hoey, D. A., Downs, M. E. & Jacobs, C. R. The mechanics of the primary cilium: An intricate structure with complex function. J. Biomech. 45, 17–26 (2012).
Pampaloni, F. et al. Thermal fluctuations of grafted microtubules provide evidence of a length-dependent persistence length. Proc. Natl Acad. Sci. USA 103, 10248–10253 (2006).
Sui, H. & Downing, K. H. Structural basis of interprotofilament interaction and lateral deformation of microtubules. Structure 18, 1022–1031 (2010).
Mandelkow, E., Schultheiss, R., Rapp, R., Müller, M. & Mandelkow, E. On the surface lattice of microtubules: Helix starts, protofilament number, seam, and handedness. J. Cell Biol. 102, 1067–1073 (1986).
Kis, A. et al. Nanomechanics of microtubules. Phys. Rev. Lett. 89, 248101 (2002).
Díaz, J. F., Barasoain, I. & Andreu, J. M. Fast kinetics of Taxol binding to microtubules. Effects of solution variables and microtubule-associated proteins. J. Biol. Chem. 278, 8407–8419 (2003).
Davis, L. J., Odde, D. J., Block, S. M. & Gross, S. P. The importance of lattice defects in katanin-mediated microtubule severing in vitro. Biophys. J. 82, 2916–2927 (2002).
Mohrbach, H. & Kulić, I. M. Motor driven microtubule shape fluctuations: Force from within the lattice. Phys. Rev. Lett. 99, 218102 (2007).
Yvon, A. M. C., Gross, D. J. & Wadsworth, P. Antagonistic forces generated by myosin II and cytoplasmic dynein regulate microtubule turnover, movement, and organization in interphase cells. Proc. Natl Acad. Sci. USA 98, 8656–8661 (2001).
Gupton, S. L., Salmon, W. C. & Waterman-Storer, C. M. Converging populations of f-actin promote breakage of associated microtubules to spatially regulate microtubule turnover in migrating cells. Curr. Biol. 12, 1891–1899 (2002).
Mandato, C. A. & Bement, W. M. Actomyosin transports microtubules and microtubules control actomyosin recruitment during Xenopus oocyte wound healing. Curr. Biol. 13, 1096–1105 (2003).
Brangwynne, C. P., Mackintosh, F. C. & Weitz, D. A. Force fluctuations and polymerization dynamics of intracellular microtubules. Proc. Natl Acad. Sci. USA 104, 16128–16133 (2007).
Bicek, A. D. et al. Anterograde microtubule transport drives microtubule bending in LLC-PK1 epithelial cells. Mol. Biol. Cell 20, 2943–2953 (2009).
Laan, L. et al. Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell 148, 502–514 (2012).
Goetz, J. G. et al. Endothelial cilia mediate low flow sensing during zebrafish vascular development. Cell Rep. 6, 799–808 (2014).
Odde, D. J., Ma, L., Briggs, A. H., Demarco, A. & Kirschner, M. W. Microtubule bending and breaking in living fibroblast cells. J. Cell Sci. 3288, 3283–3288 (1999).
Waterman-Storer, C. M. & Salmon, E. D. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J. Cell Biol. 139, 417–434 (1997).
Bicek, A. D., Tüzel, E., Kroll, D. M. & Odde, D. J. Analysis of microtubule curvature. Methods Cell Biol. 83, 237–268 (2007).
Kurachi, M., Hoshi, M. & Tashiro, H. Buckling of a single microtubule by optical trapping forces: Direct measurement of microtubule rigidity. Cell Motil. Cytoskeleton 30, 221–228 (1995).
Venier, P., Maggs, A. C., Carlier, M. F. & Pantaloni, D. Analysis of microtubule rigidity using hydrodynamic flow and thermal fluctuations. J. Biol. Chem. 269, 13353–13360 (1994).
Portran, D., Gaillard, J., Vantard, M. & Théry, M. Quantification of MAP and molecular motor activities on geometrically controlled microtubule networks. Cytoskeleton 70, 12–23 (2013).
Sangid, M. D. The physics of fatigue crack initiation. Int. J. Fatigue 57, 58–72 (2013).
Chrétien, D., Metoz, F., Verde, F., Karsenti, E. & Wade, R. H. Lattice defects in microtubules: Protofilament numbers vary within individual microtubules. J. Cell Biol. 117, 1031–1040 (1992).
Schaap, I. T., de Pablo, P. J. & Schmidt, C. F. Resolving the molecular structure of microtubules under physiological conditions with scanning force microscopy. Eur. Biophys. J. 33, 462–467 (2004).
Janson, M. E. & Dogterom, M. A bending mode analysis for growing microtubules: Evidence for a velocity-dependent rigidity. Biophys. J. 87, 2723–2736 (2004).
Brangwynne, C. P. et al. Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J. Cell Biol. 173, 733–741 (2006).
Krieg, M., Dunn, A. R. & Goodman, M. B. Mechanical control of the sense of touch by β-spectrin. Nature Cell Biol. 16, 224–233 (2014).
Dye, R. B., Flicker, P. F., Lien, D. Y. & Williams, R. C. End-stabilized microtubules observed in vitro: Stability, subunit, interchange, and breakage. Cell Motil. Cytoskeleton 21, 171–186 (1992).
Cordier, P., Tournilhac, F., Soulié-Ziakovic, C. & Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 451, 977–980 (2008).
Murphy, E. B. & Wudl, F. The world of smart healable materials. Prog. Polym. Sci. 35, 223–251 (2010).
Shelanski, M. L. Chemistry of the filaments and tubules of brain. J. Histochem. Cytochem. 21, 529–539 (1973).
Malekzadeh-Hemmat, K., Gendry, P. & Launay, J. F. Rat pancreas kinesin: Identification and potential binding to microtubules. Cell. Mol. Biol. (Noisy-le-grand) 39, 279–285 (1993).
Hyman, A. et al. Preparation of modified tubulins. Methods Enzymol. 196, 478–485 (1991).
Duffy, D. C., McDonald, J. C., Schueller, O. J. & Whitesides, G. M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 70, 4974–4984 (1998).
Doedel, E. J. AUTO-07p: Continuation and Bifurcation Software for Ordinary Differential Equations (2007); http://www.macs.hw.ac.uk/~gabriel/auto07/auto.html
Acknowledgements
We thank D. Chrétien for interesting discussions about microtubule defects and M. Dogterom and T. Salmon for bringing to our attention the seminal work of R. Williams. This work has been supported by an HFSP funding to M.T. and M.V.N. (RGY0088/2012) and ERC funding to M.T. (Starting Grant 310472).
Author information
Authors and Affiliations
Contributions
L.S. performed all experiments with the help of J.G. K.J. and L.S. conceived and performed microtubule stiffness measurements. K.J. performed numerical simulations. L.S., L.B. and M.T. designed the experiments. L.S., K.J., L.B. and M.T. analysed data. M.V.N., L.B. and M.T. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2751 kb)
Supplementary Information
Supplementary Movie 1 (MOV 2959 kb)
Supplementary Information
Supplementary Movie 2 (MOV 13935 kb)
Supplementary Information
Supplementary Movie 3 (MOV 4726 kb)
Supplementary Information
Supplementary Movie 4 (MOV 9467 kb)
Supplementary Information
Supplementary Movie 5 (MOV 13215 kb)
Supplementary Information
Supplementary Movie 6 (MOV 1332 kb)
Rights and permissions
About this article
Cite this article
Schaedel, L., John, K., Gaillard, J. et al. Microtubules self-repair in response to mechanical stress. Nature Mater 14, 1156–1163 (2015). https://doi.org/10.1038/nmat4396
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4396
This article is cited by
-
Chronic MAP4343 reverses escalated alcohol drinking in a mouse model of alcohol use disorder
Neuropsychopharmacology (2023)
-
Compressive forces stabilize microtubules in living cells
Nature Materials (2023)
-
Insights on the Role of α- and β-Tubulin Isotypes in Early Brain Development
Molecular Neurobiology (2023)
-
Mechanisms of microtubule organization in differentiated animal cells
Nature Reviews Molecular Cell Biology (2022)
-
Ciliary beating patterns map onto a low-dimensional behavioural space
Nature Physics (2022)