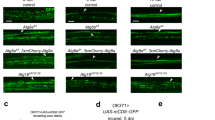
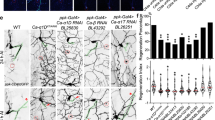
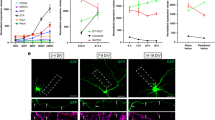
Abstract
Glial cells efficiently recognize and clear cellular debris after nervous system injury to maintain brain homeostasis, but pathways governing glial responses to neural injury remain poorly defined. We identify the Drosophila melanogaster guanine nucleotide exchange factor complex Crk/Mbc/dCed-12 and the small GTPase Rac1 as modulators of glial clearance of axonal debris. We found that Crk/Mbc/dCed-12 and Rac1 functioned in a non-redundant fashion with the Draper transmembrane receptor pathway: loss of either pathway fully suppressed clearance of axonal debris. Draper signaling was required early during glial responses, promoting glial activation, which included increased Draper and dCed-6 expression and extension of glial membranes to degenerating axons. In contrast, the Crk/Mbc/dCed-12 complex functioned at later phases, promoting glial phagocytosis of axonal debris. Our work identifies new components of the glial engulfment machinery and shows that glial activation, phagocytosis of axonal debris and termination of responses to injury are genetically separable events mediated by distinct signaling pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Cuadros, M.A. & Navascues, J. The origin and differentiation of microglial cells during development. Prog. Neurobiol. 56, 173–189 (1998).
Marín-Teva, J.L., Cuadros, M.A., Calvente, R., Almendros, A. & Navascues, J. Naturally occurring cell death and migration of microglial precursors in the quail retina during normal development. J. Comp. Neurol. 412, 255–275 (1999).
Parnaik, R., Raff, M.C. & Scholes, J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr. Biol. 10, 857–860 (2000).
Block, M.L., Zecca, L. & Hong, J.S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 8, 57–69 (2007).
Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647 (2009).
Savill, J., Dransfield, I., Gregory, C. & Haslett, C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2, 965–975 (2002).
Neumann, H., Kotter, M.R. & Franklin, R.J. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132, 288–295 (2009).
Lalancette-Hébert, M., Gowing, G., Simard, A., Weng, Y.C. & Kriz, J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 27, 2596–2605 (2007).
Fuentes-Medel, Y. et al. Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 7, e1000184 (2009).
Logan, M.A. & Freeman, M.R. The scoop on the fly brain: glial engulfment functions in Drosophila. Neuron Glia Biol. 3, 63–74 (2007).
Luo, L. & O'Leary, D.D. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 28, 127–156 (2005).
Kinchen, J.M. & Ravichandran, K.S. Journey to the grave: signaling events regulating removal of apoptotic cells. J. Cell Sci. 120, 2143–2149 (2007).
Reddien, P.W. & Horvitz, H.R. The engulfment process of programmed cell death in Caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 20, 193–221 (2004).
Hasegawa, H. et al. DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol. Cell. Biol. 16, 1770–1776 (1996).
Zhou, Z., Caron, E., Hartwieg, E., Hall, A. & Horvitz, H.R. The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev. Cell 1, 477–489 (2001).
Brugnera, E. et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat. Cell Biol. 4, 574–582 (2002).
Gumienny, T.L. et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 107, 27–41 (2001).
Zhou, Z., Hartwieg, E. & Horvitz, H.R. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell 104, 43–56 (2001).
Yu, X., Lu, N. & Zhou, Z. Phagocytic receptor CED-1 initiates a signaling pathway for degrading engulfed apoptotic cells. PLoS Biol. 6, e61 (2008).
Liu, Q.A. & Hengartner, M.O. Candidate adaptor protein CED-6 promotes the engulfment of apoptotic cells in C. elegans. Cell 93, 961–972 (1998).
Freeman, M.R., Delrow, J., Kim, J., Johnson, E. & Doe, C.Q. Unwrapping glial biology. Gcm target genes regulating glial development, diversification, and function. Neuron 38, 567–580 (2003).
Awasaki, T. et al. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron 50, 855–867 (2006).
Hoopfer, E.D. et al. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron 50, 883–895 (2006).
Williams, D.W., Kondo, S., Krzyzanowska, A., Hiromi, Y. & Truman, J.W. Local caspase activity directs engulfment of dendrites during pruning. Nat. Neurosci. 9, 1234–1236 (2006).
MacDonald, J.M. et al. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron 50, 869–881 (2006).
Doherty, J., Logan, M.A., Tasdemir, O.E. & Freeman, M.R. Ensheathing glia function as phagocytes in the adult Drosophila brain. J. Neurosci. 29, 4768–4781 (2009).
Ziegenfuss, J.S. et al. Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling. Nature 453, 935–939 (2008).
Wu, H.H. et al. Glial precursors clear sensory neuron corpses during development via Jedi-1, an engulfment receptor. Nat. Neurosci. 12, 1534–1541 (2009).
Ziegenfuss, J.S. et al. Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling. Nature 453, 935–939 (2008).
Kinchen, J.M. et al. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature 434, 93–99 (2005).
Reddien, P.W. & Horvitz, H.R. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat. Cell Biol. 2, 131–136 (2000).
Wu, Y.C., Tsai, M.C., Cheng, L.C., Chou, C.J. & Weng, N.Y. C. elegans CED-12 acts in the conserved crkII/DOCK180/Rac pathway to control cell migration and cell corpse engulfment. Dev. Cell 1, 491–502 (2001).
Logan, M.A. et al. Negative regulation of glial engulfment activity by Draper terminates glial responses to axon injury. Nat. Neurosci. 15, 722–730 (2012).
Park, D. et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450, 430–434 (2007).
Cabello, J. et al. The Wnt pathway controls cell death engulfment, spindle orientation, and migration through CED-10/Rac. PLoS Biol. 8, e1000297 (2010).
Bechmann, I. & Nitsch, R. Astrocytes and microglial cells incorporate degenerating fibers following entorhinal lesion: a light, confocal, and electron microscopical study using a phagocytosis-dependent labeling technique. Glia 20, 145–154 (1997).
Kapadia, S.E. & LaMotte, C.C. Deafferentation-induced alterations in the rat dorsal horn: I. Comparison of peripheral nerve injury vs. rhizotomy effects on presynaptic, postsynaptic, and glial processes. J. Comp. Neurol. 266, 183–197 (1987).
LaMotte, C.C. & Kapadia, S.E. Deafferentation-induced alterations in the rat dorsal horn: II. Effects of selective poisoning by pronase of the central processes of a peripheral nerve. J. Comp. Neurol. 266, 198–208 (1987).
Petersen, M.A. & Dailey, M.E. Diverse microglial motility behaviors during clearance of dead cells in hippocampal slices. Glia 46, 195–206 (2004).
Aldskogius, H. & Kozlova, E.N. Central neuron-glial and glial-glial interactions following axon injury. Prog. Neurobiol. 55, 1–26 (1998).
Leiserson, W.M., Harkins, E.W. & Keshishian, H. Fray, a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron 28, 793–806 (2000).
Ito, K., Urban, J. & Technau, G.M. Distribution, classification and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Rouxs Arch. Dev. Biol. 209, 289–307 (1995).
Ng, J. et al. Rac GTPases control axon growth, guidance and branching. Nature 416, 442–447 (2002).
Han, C., Jan, L.Y. & Jan, Y.N. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proc. Natl. Acad. Sci. USA 108, 9673–9678 (2011).
Ishimaru, S., Ueda, R., Hinohara, Y., Ohtani, M. & Hanafusa, H. PVR plays a critical role via JNK activation in thorax closure during Drosophila metamorphosis. EMBO J. 23, 3984–3994 (2004).
Acknowledgements
We thank P. Rørth (Institute of Molecular and Cell Biology, Singapore), N. Franc, S. Waddell (University of Massachusetts Medical School, Worcester), T. Awasaki (Janelia Farm), H. Hing (University of Illinois, Urbana), Y.-N. Jan (University of California, San Francisco) and Y. Nakanishi (Kanazawa University) for fly strains and antibodies. We thank L. Neukomm and A.N. Fox for critical reading of the manuscript and the entire Freeman laboratory for discussions. This work was supported by US National Institutes of Health grant RO1 NS053538 to M.R.F., and M.R.F. is an Early Career Scientist with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
J.S.Z. and M.R.F. designed the experiments; J.S.Z. conducted the majority of the experiments; J.D. performed a subset of the Rac1 studies; M.R.F. and J.S.Z. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 1428 kb)
Rights and permissions
About this article
Cite this article
Ziegenfuss, J., Doherty, J. & Freeman, M. Distinct molecular pathways mediate glial activation and engulfment of axonal debris after axotomy. Nat Neurosci 15, 979–987 (2012). https://doi.org/10.1038/nn.3135
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3135
This article is cited by
-
Lysosomal acidification dysfunction in microglia: an emerging pathogenic mechanism of neuroinflammation and neurodegeneration
Journal of Neuroinflammation (2023)
-
LC3-associated phagocytosis promotes glial degradation of axon debris after injury in Drosophila models
Nature Communications (2023)
-
Glial Draper signaling triggers cross-neuron plasticity in bystander neurons after neuronal cell death in Drosophila
Nature Communications (2023)
-
Neuronal fragile X mental retardation protein activates glial insulin receptor mediated PDF-Tri neuron developmental clearance
Nature Communications (2021)
-
Systemic loss of Sarm1 protects Schwann cells from chemotoxicity by delaying axon degeneration
Communications Biology (2020)