Abstract
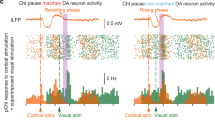
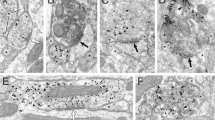
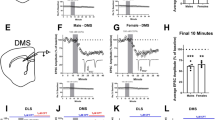
The dorsolateral striatum and cannabinoid type 1 receptor (CB1) signaling mediate habitual action learning, which is thought to require a balance of activity in the direct and indirect striatal output pathways. However, very little is known about how the high CB1–expressing striatal inhibitory microcircuitry might contribute to long-term plasticity capable of sculpting direct and indirect pathway output. Using optogenetic and molecular interrogation of striatal GABAergic microcircuits, we examined voltage-dependent long-term depression of inhibitory synapses (iLTD) onto mouse and rat medium spiny projection neurons (MSNs). The observed iLTD involved recruitment of different endocannabinoid types and showed both presynaptic and postsynaptic selectivity for MSN subtypes, ultimately resulting in a powerful disinhibition of direct pathway MSNs. These results suggest a new role for voltage states in gating circuit-specific forms of synaptic plasticity and illuminate possible circuit dynamics underlying action control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Yin, H.H. & Knowlton, B.J. Contributions of striatal subregions to place and response learning. Learn. Mem. 11, 459–463 (2004).
Hilário, M.R., Clouse, E., Yin, H.H. & Costa, R.M. Endocannabinoid signaling is critical for habit formation. Front. Integr. Neurosci. 1, 6 (2007).
Albin, R.L., Young, A.B. & Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 (1989).
Gerfen, C.R. & Surmeier, D.J. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441–466 (2011).
Mink, J.W. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch. Neurol. 60, 1365–1368 (2003).
Cui, G. et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238–242 (2013).
Uchigashima, M. et al. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J. Neurosci. 27, 3663–3676 (2007).
Tepper, J.M., Koós, T. & Wilson, C.J. GABAergic microcircuits in the neostriatum. Trends Neurosci. 27, 662–669 (2004).
Freiman, I. et al. Analysis of the effects of cannabinoids on identified synaptic connections in the caudate-putamen by paired recordings in transgenic mice. J. Physiol. (Lond.) 575, 789–806 (2006).
Winters, B.D. et al. Cannabinoid receptor 1–expressing neurons in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 109, E2717–E2725 (2012).
Wilson, C.J. & Kawaguchi, Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J. Neurosci. 16, 2397–2410 (1996).
Mahon, S. et al. Distinct patterns of striatal medium spiny neuron activity during the natural sleep-wake cycle. J. Neurosci. 26, 12587–12595 (2006).
Plenz, D. & Kitai, S.T. Up and down states in striatal medium spiny neurons simultaneously recorded with spontaneous activity in fast-spiking interneurons studied in cortex-striatum-substantia nigra organotypic cultures. J. Neurosci. 18, 266–283 (1998).
Adermark, L. & Lovinger, D.M. Combined activation of L-type Ca2+ channels and synaptic transmission is sufficient to induce striatal long-term depression. J. Neurosci. 27, 6781–6787 (2007).
Xu, W. & Lipscombe, D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J. Neurosci. 21, 5944–5951 (2001).
Adermark, L. & Lovinger, D.M. Frequency-dependent inversion of net striatal output by endocannabinoid-dependent plasticity at different synaptic inputs. J. Neurosci. 29, 1375–1380 (2009).
Maejima, T. et al. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J. Neurosci. 25, 6826–6835 (2005).
Plenz, D. When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function. Trends Neurosci. 26, 436–443 (2003).
Bracci, E. & Panzeri, S. Excitatory GABAergic effects in striatal projection neurons. J. Neurophysiol. 95, 1285–1290 (2006).
Wilson, C.J. & Groves, P.M. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J. Comp. Neurol. 194, 599–615 (1980).
Bolam, J.P., Hanley, J.J., Booth, P.A. & Bevan, M.D. Synaptic organization of the basal ganglia. J. Anat. 196, 527–542 (2000).
Kita, H., Kosaka, T. & Heizmann, C.W. Parvalbumin-immunoreactive neurons in the rat neostriatum: a light and electron microscopic study. Brain Res. 536, 1–15 (1990).
Bennett, B.D. & Bolam, J.P. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience 62, 707–719 (1994).
Kubota, Y. & Kawaguchi, Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. J. Neurosci. 20, 375–386 (2000).
Koós, T. & Tepper, J.M. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat. Neurosci. 2, 467–472 (1999).
Carter, A.G. & Sabatini, B.L. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron 44, 483–493 (2004).
Tanimura, A. et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 65, 320–327 (2010).
Egertová, M., Simon, G.M., Cravatt, B.F. & Elphick, M.R. Localization of N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) expression in mouse brain: a new perspective on N-acylethanolamines as neural signaling molecules. J. Comp. Neurol. 506, 604–615 (2008).
Tsou, K. et al. Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunohistochemistry. Neurosci. Lett. 254, 137–140 (1998).
Malone, D.T., Kearn, C.S., Chongue, L., Mackie, K. & Taylor, D.A. Effect of social isolation on CB1 and D2 receptor and fatty acid amide hydrolase expression in rats. Neuroscience 152, 265–272 (2008).
Egertová, M., Cravatt, B.F. & Elphick, M.R. Comparative analysis of fatty acid amide hydrolase and cb(1) cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience 119, 481–496 (2003).
Egertová, M., Giang, D.K., Cravatt, B.F. & Elphick, M.R. A new perspective on cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc. Biol. Sci. 265, 2081–2085 (1998).
Maccarrone, M. et al. Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J. Neurochem. 85, 1018–1025 (2003).
Maccarrone, M. et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat. Neurosci. 11, 152–159 (2008).
Puente, N. et al. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat. Neurosci. 14, 1542–1547 (2011).
Lerner, T.N. & Kreitzer, A.C. RGS4 is required for dopaminergic control of striatal LTD and susceptibility to Parkinsonian motor deficits. Neuron 73, 347–359 (2012).
Yoshida, T. et al. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J. Neurosci. 26, 4740–4751 (2006).
Narushima, M. Depolarization-induced suppression of inhibition mediated by endocannabinoids at synapses from fast-spiking interneurons to medium spiny neurons in the striatum. Eur. J. Neurosci. 24, 2246–2252 (2006).
Stern, E.A., Jaeger, D. & Wilson, C.J. Membrane potential synchrony of simultaneously recorded striatal spiny neurons in vivo. Nature 394, 475–478 (1998).
Wilson, C.J. GABAergic inhibition in the neostriatum. Prog. Brain Res. 160, 91–110 (2007).
Koós, T., Tepper, J.M. & Wilson, C.J. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J. Neurosci. 24, 7916–7922 (2004).
Schultz, W., Apicella, P. & Ljungberg, T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J. Neurosci. 13, 900–913 (1993).
Watts, A., Gritton, H.J., Sweigart, J. & Poe, G.R. Antidepressant suppression of non-REM sleep spindles and REM sleep impairs hippocampus-dependent learning while augmenting striatum-dependent learning. J. Neurosci. 32, 13411–13420 (2012).
Dang, M.T. et al. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc. Natl. Acad. Sci. USA 103, 15254–15259 (2006).
Tanahira, C. et al. Parvalbumin neurons in the forebrain as revealed by parvalbumin-Cre transgenic mice. Neurosci. Res. 63, 213–223 (2009).
Marsicano, G. et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88 (2003).
Cravatt, B.F. et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. USA 98, 9371–9376 (2001).
Caterina, M.J. et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313 (2000).
Mathur, B.N., Capik, N.A., Alvarez, V.A. & Lovinger, D.M. Serotonin induces long-term depression at corticostriatal synapses. J. Neurosci. 31, 7402–7411 (2011).
Mathur, B.N. & Deutch, A.Y. Rat meningeal and brain microvasculature pericytes co-express the vesicular glutamate transporters 2 and 3. Neurosci. Lett. 435, 90–94 (2008).
Acknowledgements
We would like to thank B. Lutz (Institute of Molecular Biology Mainz) and G. Marsicano (Institut National de la Santé et de la Recherche Médicale Bordeaux) for generously providing the Cnr1loxP/loxP mouse, and P. Pacher (National Institute on Alcohol Abuse and Alcoholism) for providing FAAH knockout mice.
Author information
Authors and Affiliations
Contributions
B.N.M. and D.M.L. designed the experiments. B.N.M. performed the experiments and analyzed the data. C.T. and N.T. generated Pvalb-Cre and CAG-mRFP-GFP mouse lines. B.N.M. and D.M.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures and Text
Supplementary Figures 1–8 and Supplementary Tables 1 and 2 (PDF 1811 kb)
Rights and permissions
About this article
Cite this article
Mathur, B., Tanahira, C., Tamamaki, N. et al. Voltage drives diverse endocannabinoid signals to mediate striatal microcircuit-specific plasticity. Nat Neurosci 16, 1275–1283 (2013). https://doi.org/10.1038/nn.3478
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3478
This article is cited by
-
Cocaine restricts nucleus accumbens feedforward drive through a monoamine-independent mechanism
Neuropsychopharmacology (2022)
-
Compulsive alcohol consumption is regulated by dorsal striatum fast-spiking interneurons
Neuropsychopharmacology (2021)
-
Cannabinoid type 1 receptors in A2a neurons contribute to cocaine-environment association
Psychopharmacology (2021)
-
TrkB-dependent disinhibition of the nucleus accumbens is enhanced by ethanol
Neuropsychopharmacology (2019)
-
Ensemble encoding of action speed by striatal fast-spiking interneurons
Brain Structure and Function (2019)