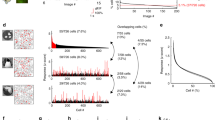
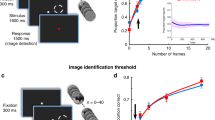
Abstract
Perceptual learning substantially improves visual discrimination and detection ability, which has been associated with visual cortical plasticity. However, little is known about the dynamic changes in neuronal response properties over the course of training. Using chronically implanted multielectrode arrays, we were able to capture day-by-day spatiotemporal dynamics of neurons in the primary visual cortex (V1) of monkeys trained to detect camouflaged visual contours. We found progressive strengthening and accelerating in both facilitation of neurons encoding the contour elements and suppression of neurons responding to the background components. The enhancement of this figure-ground contrast in V1 was closely correlated with improved behavioral performance on a daily basis. Decoding accuracy of a simple linear classifier based on V1 population responses also paralleled the animal's behavioral changes. Our results indicate that perceptual learning shapes the V1 population code to allow a more efficient readout of task-relevant information.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sasaki, Y., Nanez, J.E. & Watanabe, T. Advances in visual perceptual learning and plasticity. Nat. Rev. Neurosci. 11, 53–60 (2010).
Sagi, D. Perceptual learning in vision research. Vision Res. 51, 1552–1566 (2011).
Gilbert, C.D. & Li, W. Adult visual cortical plasticity. Neuron 75, 250–264 (2012).
Schoups, A., Vogels, R., Qian, N. & Orban, G. Practising orientation identification improves orientation coding in V1 neurons. Nature 412, 549–553 (2001).
Crist, R.E., Li, W. & Gilbert, C.D. Learning to see: Experience and attention in primary visual cortex. Nat. Neurosci. 4, 519–525 (2001).
Li, W., Piech, V. & Gilbert, C.D. Learning to link visual contours. Neuron 57, 442–451 (2008).
Hua, T. et al. Perceptual learning improves contrast sensitivity of V1 neurons in cats. Curr. Biol. 20, 887–894 (2010).
Rainer, G., Lee, H. & Logothetis, N.K. The effects of learning on the function of monkey extrastriate visual cortex. PLoS Biol. 2, e44 (2004).
Yang, T. & Maunsell, J.H.R. The effect of perceptual learning on neuronal responses in monkey visual area V4. J. Neurosci. 24, 1617–1626 (2004).
Raiguel, S., Vogels, R., Mysore, S.G. & Orban, G.A. Learning to see the difference specifically alters the most informative V4 neurons. J. Neurosci. 26, 6589–6602 (2006).
Adab, H.Z. & Vogels, R. Practicing coarse orientation discrimination improves orientation signals in macaque cortical area V4. Curr. Biol. 21, 1661–1666 (2011).
Bartolucci, M. & Smith, A.T. Attentional modulation in visual cortex is modified during perceptual learning. Neuropsychologia 49, 3898–3907 (2011).
Petrov, A.A., Dosher, B.A. & Lu, Z.L. The dynamics of perceptual learning: An incremental reweighting model. Psychol. Rev. 112, 715–743 (2005).
Law, C.-T. & Gold, J.I. Neural correlates of perceptual learning in a sensory motor, but not a sensory, cortical area. Nat. Neurosci. 11, 505–513 (2008).
Zhang, G.L., Cong, L.J., Song, Y. & Yu, C. ERP P1-N1 changes associated with Vernier perceptual learning and its location specificity and transfer. J. Vis. 13, 19 (2013).
Schwartz, S., Maquet, P. & Frith, C. Neural correlates of perceptual learning: a functional MRI study of visual texture discrimination. Proc. Natl. Acad. Sci. USA [comment] 99, 17137–17142 (2002).
Furmanski, C.S., Schluppeck, D. & Engel, S.A. Learning strengthens the response of primary visual cortex to simple patterns. Curr. Biol. 14, 573–578 (2004).
Kourtzi, Z., Betts, L.R., Sarkheil, P. & Welchman, A.E. Distributed neural plasticity for shape learning in the human visual cortex. PLoS Biol. 3, e204 (2005).
Sigman, M. et al. Top-down reorganization of activity in the visual pathway after learning a shape identification task. Neuron 46, 823–835 (2005).
Schiltz, C. et al. Neuronal mechanisms of perceptual learning: changes in human brain activity with training in orientation discrimination. Neuroimage 9, 46–62 (1999).
Mukai, I. et al. Activations in visual and attention-related areas predict and correlate with the degree of perceptual learning. J. Neurosci. 27, 11401–11411 (2007).
Yotsumoto, Y., Watanabe, T. & Sasaki, Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron 57, 827–833 (2008).
Jehee, J.F.M., Ling, S., Swisher, J.D., van Bergen, R.S. & Tong, F. Perceptual learning selectively refines orientation representations in early visual cortex. J. Neurosci. 32, 16747–16753 (2012).
Li, W., Piech, V. & Gilbert, C.D. Contour saliency in primary visual cortex. Neuron 50, 951–962 (2006).
Li, W. & Gilbert, C.D. Global contour saliency and local colinear interactions. J. Neurophysiol. 88, 2846–2856 (2002).
Nugent, A.K., Keswani, R.N., Woods, R.L. & Peli, E. Contour integration in peripheral vision reduces gradually with eccentricity. Vision Res. 43, 2427–2437 (2003).
Chen, M. et al. Incremental integration of global contours through interplay between visual cortical areas. Neuron 82, 682–694 (2014).
Li, W., Piech, V. & Gilbert, C.D. Perceptual learning and top-down influences in primary visual cortex. Nat. Neurosci. 7, 651–657 (2004).
Gu, Y. et al. Perceptual learning reduces interneuronal correlations in macaque visual cortex. Neuron 71, 750–761 (2011).
Rosenblatt, F. The perceptron: a perceiving and recognizing automaton (Report 85–460–1) (Cornell Aeronautical Laboratory, 1957).
Maass, W., Natschlager, T. & Markram, H. Real-time computing without stable states: a new framework for neural computation based on perturbations. Neural Comput. 14, 2531–2560 (2002).
Bauer, R. & Heinze, S. Contour integration in striate cortex. Classic cell responses or cooperative selection? Exp. Brain Res. 147, 145–152 (2002).
McManus, J.N.J., Li, W. & Gilbert, C.D. Adaptive shape processing in primary visual cortex. Proc. Natl. Acad. Sci. USA 108, 9739–9746 (2011).
Zipser, K., Lamme, V.A. & Schiller, P.H. Contextual modulation in primary visual cortex. J. Neurosci. 16, 7376–7389 (1996).
Roelfsema, P.R., Tolboom, M. & Khayat, P.S. Different processing phases for features, figures and selective attention in the primary visual cortex. Neuron 56, 785–792 (2007).
Poort, J. et al. The role of attention in figure-ground segregation in areas V1 and V4 of the visual cortex. Neuron 75, 143–156 (2012).
Roelfsema, P.R. & Spekreijse, H. The representation of erroneously perceived stimuli in the primary visual cortex. Neuron [see comments] 31, 853–863 (2001).
Supèr, H., Spekreijse, H. & Lamme, V.A. Two distinct modes of sensory processing observed in monkey primary visual cortex (V1). Nat. Neurosci. 4, 304–310 (2001).
Lee, T.S., Yang, C.F., Romero, R.D. & Mumford, D. Neural activity in early visual cortex reflects behavioral experience and higher-order perceptual saliency. Nat. Neurosci. 5, 589–597 (2002).
Piëch, V., Li, W., Reeke, G.N. & Gilbert, C.D. Network model of top-down influences on local gain and contextual interactions in visual cortex. Proc. Natl. Acad. Sci. USA 110, E4108–E4117 (2013).
Byers, A. & Serences, J.T. Exploring the relationship between perceptual learning and top-down attentional control. Vision Res. 74, 30–39 (2012).
Gilbert, C.D. & Li, W. Top-down influences on visual processing. Nat. Rev. Neurosci. 14, 350–363 (2013).
Shiu, L.P. & Pashler, H. Improvement in line orientation discrimination is retinally local but dependent on cognitive set. Percept. Psychophys. 52, 582–588 (1992).
Ahissar, M. & Hochstein, S. Attentional control of early perceptual learning. Proc. Natl. Acad. Sci. USA 90, 5718–5722 (1993).
Poggio, T., Fahle, M. & Edelman, S. Fast perceptual learning in visual hyperacuity. Science 256, 1018–1021 (1992).
Karni, A. & Sagi, D. The time course of learning a visual skill. Nature 365, 250–252 (1993).
Dosher, B.A. & Lu, Z.L. Mechanisms of perceptual learning. Vision Res. 39, 3197–3221 (1999).
Bejjanki, V.R., Beck, J.M., Lu, Z.L. & Pouget, A. Perceptual learning as improved probabilistic inference in early sensory areas. Nat. Neurosci. 14, 642–648 (2011).
Dosher, B.A. & Lu, Z.L. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proc. Natl. Acad. Sci. USA 95, 13988–13993 (1998).
Gold, J., Bennett, P.J. & Sekuler, A.B. Signal but not noise changes with perceptual learning. Nature 402, 176–178 (1999).
Shoham, S., Fellows, M.R. & Normann, R.A. Robust, automatic spike sorting using mixtures of multivariate t-distributions. J. Neurosci. Methods 127, 111–122 (2003).
Gretton, A., Borgwardt, K.M., Rasch, M.J., Scholkopf, B. & Smola, A. A kernel two-sample test. J. Mach. Learn. Res. 13, 723–773 (2012).
Rasch, M.J., Gretton, A., Murayama, Y., Maass, W. & Logothetis, N.K. Inferring spike trains from local field potentials. J. Neurophysiol. 99, 1461–1476 (2008).
Fisher, R.A. The use of multiple measurements in taxonomic problems. Ann. Eugen. 7, 179–188 (1936).
Acknowledgements
We thank X. Xu and F. Wang for technical assistance. This work was supported by the National Basic Research Program of China (973 Program 2014CB846101, 2011CBA00400), the National Natural Science Foundation of China (31125014, 31371109 and 30970983) and the Fundamental Research Funds for the Central Universities of China.
Author information
Authors and Affiliations
Contributions
M.C., Y.Y. and W.L. designed the experiments. Y.Y., M.C. and X.X. conducted the experiments. M.J.R., Y.Y., M.H. and S.W. analyzed the data. M.J.R. performed the population decoding analyses. M.J.R. and Y.Y. prepared the figures. M.J.R., Y.Y. and W.L. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Yan, Y., Rasch, M., Chen, M. et al. Perceptual training continuously refines neuronal population codes in primary visual cortex. Nat Neurosci 17, 1380–1387 (2014). https://doi.org/10.1038/nn.3805
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3805
This article is cited by
-
Category representation in primary visual cortex after visual perceptual learning
Cognitive Neurodynamics (2024)
-
Mapping thalamic innervation to individual L2/3 pyramidal neurons and modeling their ‘readout’ of visual input
Nature Neuroscience (2023)
-
Current directions in visual perceptual learning
Nature Reviews Psychology (2022)
-
Priority coding in the visual system
Nature Reviews Neuroscience (2022)
-
A neural correlate of perceptual segmentation in macaque middle temporal cortical area
Nature Communications (2022)